DMABlue fluorescent dyes CAS# 188860-26-6 |
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- TFB-TBOA
Catalog No.:BCC5919
CAS No.:480439-73-4
- Dihydrokainic acid
Catalog No.:BCC6556
CAS No.:52497-36-6
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- LDN 212320
Catalog No.:BCC6361
CAS No.:894002-50-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 188860-26-6 | SDF | Download SDF |
| PubChem ID | 10174012 | Appearance | Powder |
| Formula | C27H28N6O2 | M.Wt | 468.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 54 mg/mL (115.25 mM); | ||
| Chemical Name | 2-(3,4-dimethoxyphenyl)-6-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazole | ||
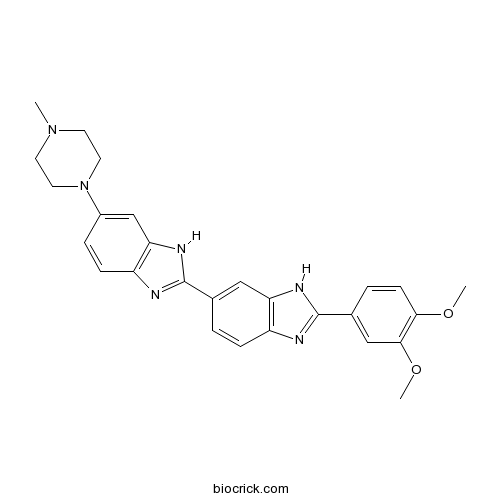
| SMILES | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=CC5=C(C=C4)N=C(N5)C6=CC(=C(C=C6)OC)OC | ||
| Standard InChIKey | BMRRDFCQNOZNNR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H28N6O2/c1-32-10-12-33(13-11-32)19-6-8-21-23(16-19)31-26(29-21)17-4-7-20-22(14-17)30-27(28-20)18-5-9-24(34-2)25(15-18)35-3/h4-9,14-16H,10-13H2,1-3H3,(H,28,30)(H,29,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | DMA is a fluorescent compound (λex=340 nm, λem=478 nm).In Vitro:The newly synthesized bisbenzimidazole derivatives DMA (6c) is evaluated for their cytotoxicity against human tumor cell lines, which are cervix carcinoma cell line (HeLa), breast carcinoma cell line (MCF7) and brain glioma cell line (U87) in comparison to Hoechst. In case of MCF7, the IC50 is observed at 5.3 μM for DMA. The IC50 determined in the case of HeLa is 3.4 μM for DMA[1]. References: | |||||

DMA Dilution Calculator

DMA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1342 mL | 10.6712 mL | 21.3424 mL | 42.6849 mL | 53.3561 mL |
| 5 mM | 0.4268 mL | 2.1342 mL | 4.2685 mL | 8.537 mL | 10.6712 mL |
| 10 mM | 0.2134 mL | 1.0671 mL | 2.1342 mL | 4.2685 mL | 5.3356 mL |
| 50 mM | 0.0427 mL | 0.2134 mL | 0.4268 mL | 0.8537 mL | 1.0671 mL |
| 100 mM | 0.0213 mL | 0.1067 mL | 0.2134 mL | 0.4268 mL | 0.5336 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C. The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably. Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. in vitro: N/A in vivo: N/A Clinical trial: N/A
- HX 630
Catalog No.:BCC6083
CAS No.:188844-52-2
- HX 531
Catalog No.:BCC6082
CAS No.:188844-34-0
- Streptozotocin
Catalog No.:BCN3834
CAS No.:18883-66-4
- 8-Glucosyl-5,7-dihydroxy-2-(1-methylpropyl)chromone
Catalog No.:BCN7505
CAS No.:188818-27-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- 3-hydroxymorindone
Catalog No.:BCN3126
CAS No.:80368-74-7
- GSK3787
Catalog No.:BCC2263
CAS No.:188591-46-0
- Cl-4AS-1
Catalog No.:BCC7780
CAS No.:188589-66-4
- TFM-4AS-1
Catalog No.:BCC6069
CAS No.:188589-61-9
- SBI-0206965
Catalog No.:BCC3984
CAS No.:1884220-36-3
- Hydroxytanshinone IIA
Catalog No.:BCN2497
CAS No.:18887-18-8
- Methyl tanshinonate
Catalog No.:BCN2553
CAS No.:18887-19-9
- Junipediol B 8-O-glucoside
Catalog No.:BCN4022
CAS No.:188894-19-1
- L 760735
Catalog No.:BCC7840
CAS No.:188923-01-5
- Cilengitide
Catalog No.:BCC3942
CAS No.:188968-51-6
- Melilotigenin C
Catalog No.:BCN1165
CAS No.:188970-21-0
- 1-(4-Hydroxy-2,2-dimethylchroman-6-yl)ethanone
Catalog No.:BCN7710
CAS No.:1890153-71-5
- Corynantheine
Catalog No.:BCN3746
CAS No.:18904-54-6
- NGB 2904
Catalog No.:BCC7435
CAS No.:189061-11-8
- [Ala92]-p16 (84-103)
Catalog No.:BCC5837
CAS No.:189064-08-2
- Oroselol
Catalog No.:BCN3907
CAS No.:1891-25-4
- Hulupinic acid
Catalog No.:BCN8019
CAS No.:1891-42-5
Paralogs and mutants show that one DMA synthase functions in iron homeostasis in rice.[Pubmed:28369596]
J Exp Bot. 2017 Mar 1;68(7):1785-1795.
Rice (Oryza sativa) secretes 2'-deoxymugineic acid (DMA) to acquire insoluble iron (Fe) from the rhizosphere. In rice, DMA is synthesized by DMA synthase 1 (OsDMAS1), a member of the aldo-keto reductase super family. We screened OsDMAS1 paralogs for DMA synthesis. None of these paralogs displayed in vitro DMA synthesis activity, suggesting that rice only harbors one functional DMAS. We further characterized OsDMAS1 mutant plants. We failed to screen homozygous knock-out plants (DMAs-1), so we characterized DMAS knock-down plants (DMAs-kd1 and DMAs-kd2). Under Fe-deficient conditions, DMAs-kd1 plants were more chlorotic compared to the wild-type (WT) plants, and the expression of OsNAS3, OsYSL2, OsIRT1, and OsIRO2 was significantly up-regulated in the DMAs-kd1 mutant, indicating that metal homeostasis was significantly disturbed. The secretion of DMA in DMAs-kd1 was not significantly reduced. The DMAs-kd1 plants accumulated less Fe in their roots compared to WT plants when grown with 10 muM FeSO4. The DMAs-kd1 plants accumulated more Zn in their roots compared to WT plants under Fe-deficient, Fe-EDTA, and FeSO4 conditions. In both dehusked rice seeds (brown rice) and polished rice, no differences were observed for Fe, Cu, or Mn accumulation, whereas DMAs-kd1 seeds significantly accumulated more Zn in brown rice. Our data suggests that rice only harbors one functional gene for DMA synthesis. In addition, the knock-down of OsDMAS1 significantly up-regulates the genes involved in Fe uptake and homeostasis.
Antioxidant effect of quercetin in an extender containing DMA or glycerol on freezing capacity of goat semen.[Pubmed:28279680]
Cryobiology. 2017 Apr;75:15-20.
This study was performed to evaluate the effectiveness of quercetin as a non-enzymatic antioxidant in combination with glycerol or Dimethylacetamide (DMA), on freezability of goat semen. Ejaculates from four healthy mature Mahabadi goats were collected using an artificial vagina. After primary processing, semen was pooled and extended by egg yolk based extender supplemented with different concentrations of quercetin (10 or 20 muM) along with 5% glycerol or DMA. The extended semen was frozen and sperm motility parameters, viability, abnormality, membrane integrity and lipid peroxidation were assessed after thawing. Results showed that sperm viability, total motility, progressive motility, straightness (STR) and linearity (LIN) were higher (P < 0.05), and abnormality percentage and MDA concentration were lower (P < 0.05) in extender containing DMA. Similarly, higher (P < 0.05) total motility, progressive motility, viability and membrane integrity along with lower (P < 0.05) MDA level were noted in Q10 group. The lowest (P < 0.05) MDA level was observed in DMA extender containing moderate level of quercetin (Q10D). Also the STR was higher (P < 0.05) in Q10D compared to Q10G and Q20G groups. In conclusion, supplementation of extender with 10 muM quercetin in combination with DMA improves the goat sperm motion kinetics and suppresses lipid peroxidation after freezing and thawing. Furthermore, DMA is more effective cryoprotectant for the freezing of goat sperm.
Experimental Determination of Zinc Isotope Fractionation in Complexes with the Phytosiderophore 2'-Deoxymugeneic Acid (DMA) and Its Structural Analogues, and Implications for Plant Uptake Mechanisms.[Pubmed:27750003]
Environ Sci Technol. 2017 Jan 3;51(1):98-107.
The stable isotope signatures of zinc and other metals are increasingly used to study plant and soil processes. Complexation with phytosiderophores is a key reaction and understanding the controls of isotope fractionation is central to such studies. Here, we investigated isotope fractionation during complexation of Zn(2+) with the phytosiderophore 2'-deoxymugeneic acid (DMA), and with three commercially available structural analogues of DMA: EDTA, TmDTA, and CyDTA. We used ion exchange chromatography to separate free and complexed zinc, and identified appropriate cation exchange resins for the individual systems. These were Chelex-100 for EDTA and CyDTA, Amberlite CG50 for TmDTA and Amberlite IR120 for DMA. With all the ligands we found preferential partitioning of isotopically heavy zinc in the complexed form, and the extent of fractionation was independent of the Zn:ligand ratio used, indicating isotopic equilibrium and that the results were not significantly affected by artifacts during separation. The fractionations (in per thousand) were +0.33 +/- 0.07 (1sigma, n = 3), + 0.45 +/- 0.02 (1sigma, n = 2), + 0.62 +/- 0.05 (1sigma, n = 3) and +0.30 +/- 0.07 (1sigma, n = 4) for EDTA, TmDTA, CyDTA, and DMA, respectively. Despite the similarity in Zn-coordinating donor groups, the fractionation factors are significantly different and extent of fractionation seems proportional to the complexation stability constant. The extent of fractionation with DMA agreed with observed fractionations in zinc uptake by paddy rice in field experiments, supporting the possible involvement of DMA in zinc uptake by rice.
Arsenic Methyltransferase is Involved in Arsenosugar Biosynthesis by Providing DMA.[Pubmed:28076949]
Environ Sci Technol. 2017 Feb 7;51(3):1224-1230.
Arsenic is an ubiquitous toxic element in the environment, and organisms have evolved different arsenic detoxification strategies. Studies on arsenic biotransformation mechanisms have mainly focused on arsenate (As(V)) reduction, arsenite (As(III)) oxidation, and arsenic methylation; little is known, however, about the pathway for the biosynthesis of arsenosugars, which are significant arsenic transformation products. Here, the involvement of As(III) S-Adenosylmethionine methyltransferase (ArsM) in arsenosugar synthesis is demonstrated for the first time. Synechocystis sp. PCC 6803 incubated with As(III) or monomethylarsonic acid (MMA(V)) produced dimethylarsinic acid (DMA(V)) and arsenosugars, as determined by high performance liquid chromatography-inductively coupled plasma mass spectrometry (HPLC/ICPMS). Arsenosugars were also detected in the cells when they were exposed to DMA(V). A mutant strain Synechocystis DeltaarsM was constructed by disrupting arsM in Synechocystis sp. PCC 6803. Methylation of arsenic species was not observed in the mutant strain after exposure to arsenite or MMA(V); when Synechocystis DeltaarsM was incubated with DMA(V), arsenosugars were detected in the cells. These results suggest that ArsM is a required enzyme for the methylation of inorganic arsenicals, but not required for the synthesis of arsenosugars from DMA, and that DMA is the precursor of arsenosugar biosynthesis. The findings will stimulate more studies on the biosynthesis of complex organoarsenicals, and lead to a better understanding of the bioavailability and function of the organoarsenicals in biological systems.


