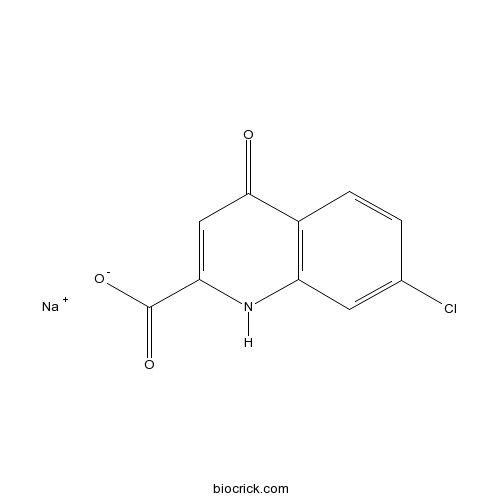
7-Chlorokynurenic acid sodium saltCAS# 1263094-00-3 |
- NSC 23766
Catalog No.:BCC1149
CAS No.:1177865-17-6
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- EHop-016
Catalog No.:BCC5022
CAS No.:1380432-32-5
- ZCL278
Catalog No.:BCC3665
CAS No.:587841-73-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1263094-00-3 | SDF | Download SDF |
| PubChem ID | 52974249 | Appearance | Powder |
| Formula | C10H5ClNNaO3 | M.Wt | 245.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 50 mg/mL (203.59 mM; Need ultrasonic) DMSO : ≥ 33.67 mg/mL (137.10 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | sodium;7-chloro-4-oxo-1H-quinoline-2-carboxylate | ||
| SMILES | C1=CC2=C(C=C1Cl)NC(=CC2=O)C(=O)[O-].[Na+] | ||
| Standard InChIKey | IFZYIORLNGNLEI-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C10H6ClNO3.Na/c11-5-1-2-6-7(3-5)12-8(10(14)15)4-9(6)13;/h1-4H,(H,12,13)(H,14,15);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sodium salt of 7-Chlorokynurenic acid, an NMDA receptor antagonist acting at the glycine site. Potent competitive inhibitor of L-glutamate transport into synaptic vesicles. |

7-Chlorokynurenic acid sodium salt Dilution Calculator

7-Chlorokynurenic acid sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0718 mL | 20.3591 mL | 40.7183 mL | 81.4365 mL | 101.7957 mL |
| 5 mM | 0.8144 mL | 4.0718 mL | 8.1437 mL | 16.2873 mL | 20.3591 mL |
| 10 mM | 0.4072 mL | 2.0359 mL | 4.0718 mL | 8.1437 mL | 10.1796 mL |
| 50 mM | 0.0814 mL | 0.4072 mL | 0.8144 mL | 1.6287 mL | 2.0359 mL |
| 100 mM | 0.0407 mL | 0.2036 mL | 0.4072 mL | 0.8144 mL | 1.018 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DL-AP4 Sodium salt
Catalog No.:BCC7759
CAS No.:1263093-79-3
- Paromomycin Sulfate
Catalog No.:BCC4694
CAS No.:1263-89-4
- NCX 466
Catalog No.:BCC6219
CAS No.:1262956-64-8
- Spautin-1
Catalog No.:BCC6420
CAS No.:1262888-28-7
- CCT241533
Catalog No.:BCC1462
CAS No.:1262849-73-9
- 25-Hydroxy VD2-D6
Catalog No.:BCC1305
CAS No.:1262843-46-8
- Trigonosin F
Catalog No.:BCN6403
CAS No.:1262842-73-8
- Penitrem A
Catalog No.:BCC7957
CAS No.:12627-35-9
- Benzoylmesaconine hydrochloride
Catalog No.:BCN5399
CAS No.:126266-38-4
- GS967
Catalog No.:BCC6401
CAS No.:1262618-39-2
- Assamicadine
Catalog No.:BCN1957
CAS No.:126260-96-6
- Fumitremorgin B
Catalog No.:BCN6453
CAS No.:12626-17-4
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- Pinobanksin 3-O-propanoate
Catalog No.:BCN7737
CAS No.:126394-70-5
- Nortropinyl cinnamate
Catalog No.:BCN1891
CAS No.:126394-79-4
- Colistin Sulfate
Catalog No.:BCC4653
CAS No.:1264-72-8
- SR 12813
Catalog No.:BCC7530
CAS No.:126411-39-0
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- Pyrrolam A
Catalog No.:BCN2040
CAS No.:126424-76-8
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- (R)-CPP
Catalog No.:BCC6581
CAS No.:126453-07-4
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
Substituted quinolines as inhibitors of L-glutamate transport into synaptic vesicles.[Pubmed:9776380]
Neuropharmacology. 1998 Jul;37(7):839-46.
This study investigated the structure-activity relationships and kinetic properties of a library of kynurenate analogues as inhibitors of 3H-L-glutamate transport into rat forebrain synaptic vesicles. The lack of inhibitory activity observed with the majority of the monocyclic pyridine derivatives suggested that the second aromatic ring of the quinoline-based compounds played a significant role in binding to the transporter. A total of two kynurenate derivatives, xanthurenate and 7-chloro-kynurenate, differing only in the carbocyclic ring substituents, were identified as potent competitive inhibitors, exhibiting Ki values of 0.19 and 0.59 mM, respectively. The Km value for L-glutamate was found to be 2.46 mM. Parallel experiments demonstrated that while none of the kynurenate analogues tested effectively inhibited the synaptosomal transport of 3H-D-aspartate, some cross-reactivity was observed with the EAA ionotropic receptors. Molecular modeling studies were carried out with the identified inhibitors and glutamate in an attempt to preliminarily define the pharmacophore of the vesicular transporter. It is hypothesized that the ability of the kynurenate analogues to bind to the transporter may be tied to the capacity of the quinoline carbocyclic ring to mimic the negative charge of the gamma-carboxylate of glutamate. A total of two low energy solution conformers of glutamate were identified that exhibited marked functional group overlap with the most potent inhibitor, xanthurenate. These results help to further refine the pharmacological specificity of the glutamate binding site on the vesicular transporter and identify a series of inhibitors with which to investigate transporter function.
Behavioral and neurochemical actions of the strychnine-insensitive glycine receptor antagonist, 7-chlorokynurenate, in rats.[Pubmed:7498252]
Eur J Pharmacol. 1995 Jun 23;280(1):37-45.
The present study investigated if blockade of the modulatory glycine receptor of the NMDA receptor complex influences the expression of behavior (sniffing stereotypy and locomotion) and dopamine metabolism in rats as it has been shown for NMDA receptor antagonists. The glycine receptor antagonist, 7-chlorokynurenate (7-chloro-4-hydroxyquinoline-2-carboxylic acid), induced a dose-dependent sniffing stereotypy but had no effect on locomotion when it was given i.c.v. The glycine receptor agonist, D-cycloserine (D-4-amino-3-isoxazolidinone), antagonized the sniffing stereotypy. 7-Chlorokynurenate had no influence on dopamine metabolism in the striatum and the nucleus accumbens, but moderately decreased the metabolism in the prefrontal cortex. Comparison of behavioral and neurochemical outcomes suggests that the failure to induce locomotion correlates with the unchanged dopamine metabolism in the basal ganglia, while sniffing stereotypy does not. These results show that blockade of the glycine receptor of the NMDA receptor complex induces a behavioral and neurochemical profile similar to that of competitive NMDA receptor antagonists.


