ZCL278Selective Cdc42 inhibitor CAS# 587841-73-4 |
- ML 141
Catalog No.:BCC8092
CAS No.:71203-35-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 587841-73-4 | SDF | Download SDF |
| PubChem ID | 1791111 | Appearance | Powder |
| Formula | C21H19BrClN5O4S2 | M.Wt | 584.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (85.49 mM; Need ultrasonic) | ||
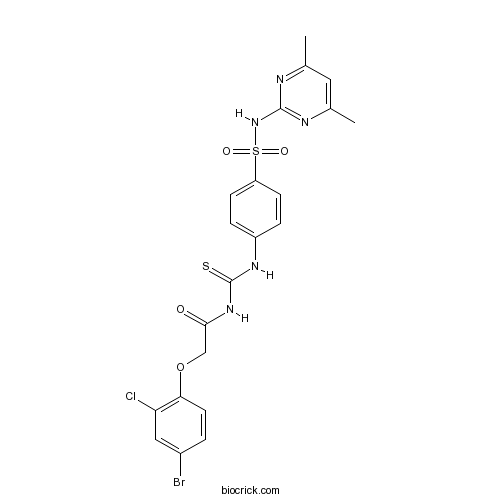
| Chemical Name | 2-(4-bromo-2-chlorophenoxy)-N-[[4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl]carbamothioyl]acetamide | ||
| SMILES | CC1=CC(=NC(=N1)NS(=O)(=O)C2=CC=C(C=C2)NC(=S)NC(=O)COC3=C(C=C(C=C3)Br)Cl)C | ||
| Standard InChIKey | XKZDWYDHEBCGCG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H19BrClN5O4S2/c1-12-9-13(2)25-20(24-12)28-34(30,31)16-6-4-15(5-7-16)26-21(33)27-19(29)11-32-18-8-3-14(22)10-17(18)23/h3-10H,11H2,1-2H3,(H,24,25,28)(H2,26,27,29,33) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of Cdc42. Targets the binding site of the Cdc42 guanine nucleotide exchange factor, intersectin (ITSN). Inhibits Cdc42-mediated cellular effects, including microspike formation in 3T3 fibroblasts and neuronal branching in primary neonatal cortical neurons. Also suppresses cell motility and migration in PC3 cells, without cytotoxic effects. | |||||

ZCL278 Dilution Calculator

ZCL278 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7097 mL | 8.5486 mL | 17.0972 mL | 34.1945 mL | 42.7431 mL |
| 5 mM | 0.3419 mL | 1.7097 mL | 3.4194 mL | 6.8389 mL | 8.5486 mL |
| 10 mM | 0.171 mL | 0.8549 mL | 1.7097 mL | 3.4194 mL | 4.2743 mL |
| 50 mM | 0.0342 mL | 0.171 mL | 0.3419 mL | 0.6839 mL | 0.8549 mL |
| 100 mM | 0.0171 mL | 0.0855 mL | 0.171 mL | 0.3419 mL | 0.4274 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ZCL278 is a selective inhibitor of Cdc42 with Kd value of 11.4 μM [1].
Cell division control protein 42 homolog (Cdc42) is a small GTPase that belongs to the Rho family and plays an important role in regulating diverse cellular functions including cell morphology, endocytosis, migration, and cell cycle progression [2].
ZCL278 is a potent Cdc42 inhibitor and has a different selectivity with the reported Cdc42 inhibitor ML141. When tested with human metastatic prostate cancer PC-3 cells, ZCL278 showed inhibitory function on Rac/Cdc42 phosphorylation and the function increasing as the more-treated time. In cortical neurons, ZCL278 treatment suppressed neuronal branch number and inhibited growth cone motility at the dose of 50 μM for 5 or 10 min. Treated serum-starved Swiss 3T3 fibroblasts Cdc42 activator following administration of ZCL278 at the dose of 50 μM for 1 h exhibited a significant decrease (nearly 80%) in GTP-Cdc42 and disrupted perinuclear distribution of active Cdc42. [1]. When tested with rat cerebellar granule neurons (CGNs), pre-treated with ZCL278 before exposed to NaAsO2 increased cell viability in a dose-dependent manner (20, 50 and 100μM) [3].
References:
[1]. Friesland, A., et al., Small molecule targeting Cdc42-intersectin interaction disrupts Golgi organization and suppresses cell motility. Proc Natl Acad Sci U S A, 2013. 110(4): p. 1261-6.
[2]. Selamat, W., et al., The Cdc42 Effector Kinase PAK4 Localizes to Cell-Cell Junctions and Contributes to Establishing Cell Polarity. PLoS One, 2015. 10(6): p. e0129634.
[3]. Liu, X., et al., Neuroglobin Plays a Protective Role in Arsenite-Induced Cytotoxicity by Inhibition of Cdc42 and Rac1GTPases in Rat Cerebellar Granule Neurons. Cell Physiol Biochem, 2015. 36(4): p. 1613-1627.
- Alpha-Belladonnine
Catalog No.:BCN1894
CAS No.:5878-33-1
- Pinostilbenoside
Catalog No.:BCN5799
CAS No.:58762-96-2
- Haplopine
Catalog No.:BCN3921
CAS No.:5876-17-5
- Meranzin hydrate
Catalog No.:BCN5798
CAS No.:5875-49-0
- Proparacaine HCl
Catalog No.:BCC5073
CAS No.:5875-06-9
- Licochalcone B
Catalog No.:BCN6333
CAS No.:58749-23-8
- Licochalcone A
Catalog No.:BCN6332
CAS No.:58749-22-7
- H-D-Ser-OMe.HCl
Catalog No.:BCC3098
CAS No.:5874-57-7
- 16-Oxoprometaphanine
Catalog No.:BCN5797
CAS No.:58738-31-1
- H-D-Cha-OH
Catalog No.:BCC2662
CAS No.:58717-02-5
- Dihydrokavain
Catalog No.:BCN2677
CAS No.:587-63-3
- 3,4-Dichloro-Phe-OH
Catalog No.:BCC2636
CAS No.:587-56-4
- C7280948
Catalog No.:BCC6443
CAS No.:587850-67-7
- ABT 724 trihydrochloride
Catalog No.:BCC7293
CAS No.:587870-77-7
- KU 55933
Catalog No.:BCC2475
CAS No.:587871-26-9
- Benzalazine
Catalog No.:BCC8843
CAS No.:588-68-1
- Toosendanin
Catalog No.:BCN1007
CAS No.:58812-37-6
- SB 297006
Catalog No.:BCC6129
CAS No.:58816-69-6
- [Leu5]-Enkephalin
Catalog No.:BCC5831
CAS No.:58822-25-6
- Secoxyloganin
Catalog No.:BCN5800
CAS No.:58822-47-2
- 9-Oxonerolidol
Catalog No.:BCN5801
CAS No.:58865-88-6
- Ophiopogonanone E
Catalog No.:BCN6625
CAS No.:588706-66-5
- Ophiopogonanone F
Catalog No.:BCN6409
CAS No.:588706-67-6
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
Rac1 and Cdc42 Play Important Roles in Arsenic Neurotoxicity in Primary Cultured Rat Cerebellar Astrocytes.[Pubmed:26231544]
Biol Trace Elem Res. 2016 Mar;170(1):173-82.
This study aimed to explore whether Rac1 and Cdc42, representative members of Ras homologue guanosine triphosphatases (Rho GTPases), are involved in neurotoxicity induced by arsenic exposure in rat nervous system. Expressions of Rac1 and Cdc42 in rat cerebellum and cerebrum exposed to different doses of NaAsO2 (Wistar rats drank 0, 2, 10, and 50 mg/L NaAsO2 water for 3 months) were examined. Both Rac1 and Cdc42 expressions increased significantly in a dose-dependent manner in cerebellum (P < 0.01) by Western blot and immunohistochemistry assay, but in cerebrum, Rac1 and Cdc42 expressions only in 2 mg/L exposure groups were significantly higher than those in control groups (P < 0.01). Five to 50 muM NaAsO2 decreased cell viability in a dose-dependent manner in primary cultured rat astrocytes, whereas 1 muM NaAsO2 increased the cell viability in these cells. Rac1 inhibitor, NSC23766, decreased NaAsO2-induced apoptosis and increased the cell viability in primary cultured rat cerebellar astrocytes exposed to 30 muM NaAsO2. Cdc42 inhibitor, ZCL278, increased cell viability in the cells exposed to 30 muM NaAsO2. Taken together, our current studies in vivo and in vitro indicate that activations of Rac1 and Cdc42 play a very important role in arsenic neurotoxicity in rat cerebellum, providing a new insight into arsenic neurotoxicity.
Neuroglobin Plays a Protective Role in Arsenite-Induced Cytotoxicity by Inhibition of Cdc42 and Rac1GTPases in Rat Cerebellar Granule Neurons.[Pubmed:26160017]
Cell Physiol Biochem. 2015;36(4):1613-27.
BACKGROUND AND AIMS: We have previously shown that neuroglobin (Ngb) expression can be regulated by sodium arsenite (NaAsO2) exposure in rat cerebellar granule neurons (CGNs). However, the precise molecular mechanisms of Ngb action are largely unknown. Ras homolog (Rho) guanosine triphosphatases (Rho GTPases) are involved in the regulation of a number of cellular processes, including cell cytotoxicity. It has been reported that Ngb can act as a guanine nucleotide dissociation inhibitior (GDI) role to inactivate Rho GTPases. Therefore, we investigated Rho GTPases activation induced by NaAsO2 exposure in rat CGNs and effects of Rho GTPases activation on the cells. We also investigated the role of Ngb in this process. METHODS: Primary cultures of CGNs were prepared from 7-day-old Wistar rat pups. The cytotoxic effects of NaAsO2 on CGNs were evaluated using the Cell Counting Kit-8 assay and TUNEL staining. RNA interference technology was used to silence Ngb, and the subsequent effects were evaluated by quantitative RT-PCR and Western blot. Cdc42 and Rac1 activation were measured by pull-down assay and Western blot. RESULTS: NaAsO2 induced cytotoxicity in rat CGNs, increased GTP-bound form of Cdc42 and Rac1 GTPases in the cells. Furthermore, inhibition of Cdc42 or Rac1 activity using the inhibitor ZCL278 or NSC23766 decreased apoptosis and increased cell viability in the cells exposed to NaAsO2. Using siRNA-mediated knockdown, we show that NaAsO2-induced cytotoxicity was exacerbated, activation of Cdc42 (GTP-Cdc42) and Rac1 (GTP-Rac1) was increased in Ngb RNA silencing cells. CONCLUSIONS: cytotoxic effects of NaAsO2 on rat CGNs is induced at least partly by Cdc42 and Rac1 activation, and Ngb can inhibit Cdc42 and Rac1 activation to play protective role in rat CGNs exposed to NaAsO2.
Small molecule targeting Cdc42-intersectin interaction disrupts Golgi organization and suppresses cell motility.[Pubmed:23284167]
Proc Natl Acad Sci U S A. 2013 Jan 22;110(4):1261-6.
Signaling through the Rho family of small GTPases has been intensely investigated for its crucial roles in a wide variety of human diseases. Although RhoA and Rac1 signaling pathways are frequently exploited with the aid of effective small molecule modulators, studies of the Cdc42 subclass have lagged because of a lack of such means. We have applied high-throughput in silico screening and identified compounds that are able to fit into the surface groove of Cdc42, which is critical for guanine nucleotide exchange factor binding. Based on the interaction between Cdc42 and intersectin (ITSN), a specific Cdc42 guanine nucleotide exchange factor, we discovered compounds that rendered ITSN-like interactions in the binding pocket. By using in vitro binding and imaging as well as biochemical and cell-based assays, we demonstrated that ZCL278 has emerged as a selective Cdc42 small molecule modulator that directly binds to Cdc42 and inhibits its functions. In Swiss 3T3 fibroblast cultures, ZCL278 abolished microspike formation and disrupted GM130-docked Golgi structures, two of the most prominent Cdc42-mediated subcellular events. ZCL278 reduces the perinuclear accumulation of active Cdc42 in contrast to NSC23766, a selective Rac inhibitor. ZCL278 suppresses Cdc42-mediated neuronal branching and growth cone dynamics as well as actin-based motility and migration in a metastatic prostate cancer cell line (i.e., PC-3) without disrupting cell viability. Thus, ZCL278 is a small molecule that specifically targets Cdc42-ITSN interaction and inhibits Cdc42-mediated cellular processes, thus providing a powerful tool for research of Cdc42 subclass of Rho GTPases in human pathogenesis, such as those of cancer and neurological disorders.
Identification and Characterization of a Novel Broad-Spectrum Virus Entry Inhibitor.[Pubmed:26912630]
J Virol. 2016 Apr 14;90(9):4494-4510.
UNLABELLED: Virus entry into cells is a multistep process that often requires the subversion of subcellular machineries. A more complete understanding of these steps is necessary to develop new antiviral strategies. While studying the potential role of the actin network and one of its master regulators, the small GTPase Cdc42, during Junin virus (JUNV) entry, we serendipitously uncovered the small molecule ZCL278, reported to inhibit Cdc42 function as an entry inhibitor for JUNV and for vesicular stomatitis virus, lymphocytic choriomeningitis virus, and dengue virus but not for the nonenveloped poliovirus. Although ZCL278 did not interfere with JUNV attachment to the cell surface or virus particle internalization into host cells, it prevented the release of JUNV ribonucleoprotein cores into the cytosol and decreased pH-mediated viral fusion with host membranes. We also identified SVG-A astroglial cell-derived cells to be highly permissive for JUNV infection and generated new cell lines expressing fluorescently tagged Rab5c or Rab7a or lacking Cdc42 using clustered regularly interspaced short palindromic repeat (CRISPR)-caspase 9 (Cas9) gene-editing strategies. Aided by these tools, we uncovered that perturbations in the actin cytoskeleton or Cdc42 activity minimally affect JUNV entry, suggesting that the inhibitory effect of ZCL278 is not mediated by ZCL278 interfering with the activity of Cdc42. Instead, ZCL278 appears to redistribute viral particles from endosomal to lysosomal compartments. ZCL278 also inhibited JUNV replication in a mouse model, and no toxicity was detected. Together, our data suggest the unexpected antiviral activity of ZCL278 and highlight its potential for use in the development of valuable new tools to study the intracellular trafficking of pathogens. IMPORTANCE: The Junin virus is responsible for outbreaks of Argentine hemorrhagic fever in South America, where 5 million people are at risk. Limited options are currently available to treat infections by Junin virus or other viruses of the Arenaviridae, making the identification of additional tools, including small-molecule inhibitors, of great importance. How Junin virus enters cells is not yet fully understood. Here we describe new cell culture models in which the cells are susceptible to Junin virus infection and to which we applied CRISPR-Cas9 genome engineering strategies to help characterize early steps during virus entry. We also uncovered ZCL278 to be a new antiviral small molecule that potently inhibits the cellular entry of the Junin virus and other enveloped viruses. Moreover, we show that ZCL278 also functions in vivo, thereby preventing Junin virus replication in a mouse model, opening the possibility for the discovery of ZCL278 derivatives of therapeutic potential.


