3,4-Dichloro-Phe-OHCAS# 587-56-4 |
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

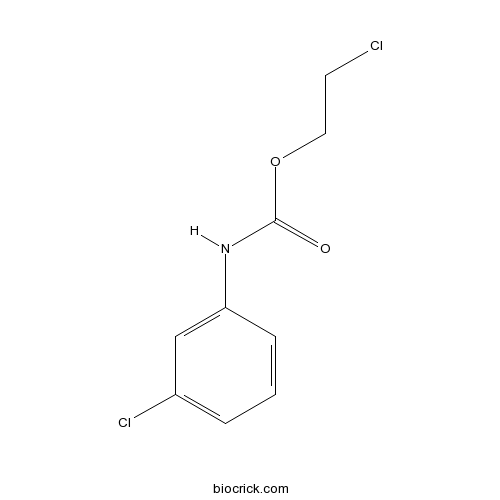
| Cas No. | 587-56-4 | SDF | Download SDF |
| PubChem ID | 11483 | Appearance | Powder |
| Formula | C9H9Cl2NO2 | M.Wt | 234.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-chloroethyl N-(3-chlorophenyl)carbamate | ||
| SMILES | C1=CC(=CC(=C1)Cl)NC(=O)OCCCl | ||
| Standard InChIKey | GYJQWEIGUGMFMU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H9Cl2NO2/c10-4-5-14-9(13)12-8-3-1-2-7(11)6-8/h1-3,6H,4-5H2,(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3,4-Dichloro-Phe-OH Dilution Calculator

3,4-Dichloro-Phe-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.272 mL | 21.3602 mL | 42.7204 mL | 85.4409 mL | 106.8011 mL |
| 5 mM | 0.8544 mL | 4.272 mL | 8.5441 mL | 17.0882 mL | 21.3602 mL |
| 10 mM | 0.4272 mL | 2.136 mL | 4.272 mL | 8.5441 mL | 10.6801 mL |
| 50 mM | 0.0854 mL | 0.4272 mL | 0.8544 mL | 1.7088 mL | 2.136 mL |
| 100 mM | 0.0427 mL | 0.2136 mL | 0.4272 mL | 0.8544 mL | 1.068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
3,4-Dichloro-Phe-OH
- 2-C-Methyl-D-erythritol
Catalog No.:BCC8570
CAS No.:58698-37-6
- Boc-Cys(Acm)-ONp
Catalog No.:BCC3375
CAS No.:58651-76-6
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- Boc-ON
Catalog No.:BCC2797
CAS No.:58632-95-4
- H- ß-HoGlu-OH.HCl
Catalog No.:BCC3232
CAS No.:58610-41-6
- m-Anisic acid
Catalog No.:BCC9015
CAS No.:586-38-9
- Anagrelide HCl
Catalog No.:BCC2306
CAS No.:58579-51-4
- Saikosaponin B4
Catalog No.:BCN8516
CAS No.:58558-09-1
- Saikosaponin B1
Catalog No.:BCN5917
CAS No.:58558-08-0
- Losmapimod
Catalog No.:BCC5368
CAS No.:585543-15-3
- Confluentin
Catalog No.:BCN5795
CAS No.:585534-03-8
- Schisantherin A
Catalog No.:BCN1024
CAS No.:58546-56-8
- Dihydrokavain
Catalog No.:BCN2677
CAS No.:587-63-3
- H-D-Cha-OH
Catalog No.:BCC2662
CAS No.:58717-02-5
- 16-Oxoprometaphanine
Catalog No.:BCN5797
CAS No.:58738-31-1
- H-D-Ser-OMe.HCl
Catalog No.:BCC3098
CAS No.:5874-57-7
- Licochalcone A
Catalog No.:BCN6332
CAS No.:58749-22-7
- Licochalcone B
Catalog No.:BCN6333
CAS No.:58749-23-8
- Proparacaine HCl
Catalog No.:BCC5073
CAS No.:5875-06-9
- Meranzin hydrate
Catalog No.:BCN5798
CAS No.:5875-49-0
- Haplopine
Catalog No.:BCN3921
CAS No.:5876-17-5
- Pinostilbenoside
Catalog No.:BCN5799
CAS No.:58762-96-2
- Alpha-Belladonnine
Catalog No.:BCN1894
CAS No.:5878-33-1
- ZCL278
Catalog No.:BCC3665
CAS No.:587841-73-4
Evaluation of the (1,3)-beta-D-glucan assay for the diagnosis of neonatal invasive yeast infections.[Pubmed:28371838]
Med Mycol. 2018 Jan 1;56(1):78-87.
Most newborns in the neonatal intensive care unit (NICU) are premature and at risk of invasive fungal infections (IFIs). Invasive yeast infections (IYIs) are the most common fungal infections in this population. These infections are difficult to diagnose because symptoms are nonspecific, and the sensitivity of blood cultures is low. The serum (1,3)-beta-D-glucan (BDG) assay provides a reliable marker for the diagnosis of IFIs in adults with haematological malignancies. We assessed the diagnostic performance of this test in neonatal IYIs and its contribution to the monitoring of antifungal treatment. A retrospective study was performed in the NICU of the French University Hospital of Amiens from February 2012 to February 2014. Forty-seven neonates (33 males, 14 females) with a median gestational age of 30 weeks (IQR: 27-31) and median birth weight of 1200 g (IQR: 968-1700) were included and divided into three groups: 21 control neonates (CTRL), 20 neonates with probable IYI (PB), and six with proven IYI (PV). Median BDG levels were significantly higher in the global IYI group (PB + PV): 149 pg/ml (IQR: 85-364) vs. CTRL group: 39 pg/ml (IQR: 20-94) (P < .001). The optimal cut-off was 106 pg/ml (sensitivity 61.5%; specificity 81%). BDG levels decreased with antifungal treatment. BDG was detectable in cerebrospinal fluid, but the interest of this for diagnostic purposes remains unclear. Our results suggest that the BDG assay may be useful for the early identification of IYIs in neonates and for monitoring antifungal therapy efficacy.
Angiopoietin-like protein 3 and 4 expression 4 and their serum levels in hepatocellular carcinoma.[Pubmed:28371666]
Cytokine. 2017 Aug;96:75-86.
BACKGROUND: Hepatocellular carcinoma (HCC) is the 6th most common cancer and the 3rd leading cause of cancer causing death allover the world. The aim of this research to explore the clinical relevance of blood angiopoietin-like protein-3 (ANGPTL3) and ANGPTL4 expression and their proteins levels as non invasive biomarkers in cirrhotic and HCC patients and their influence on the clinicopathological features of HCC. MATERIAL AND METHODS: This work comprised 200 patients with chronic hepatitis (120 cases complicated with cirrhosis, 80 patients with primary HCC) and 100 controls. circulating ANGPTL3 and ANGPTL4 expression was estimated by real-time polymerase chain reaction (RT-PCR). ANGPTL3 and ANGPTL4 protein levels were determined by enzyme-linked immunosorbent assay (ELISA). RESULTS: The circulating ANGPTL3 and ANGPTL 4 expression was significantly elevated in HCC cases compared to chronic hepatitis patients and controls. There were much more serum ANGPTL3 and ANGPTL4 values in HCC and chronic hepatitis patients as compared to controls, but we couldn't detect this significance between chronic hepatitis and HCC cases as regards ANGPTL4. By Multiple stepwise linear regression analysis, an increased ANGPTL3 expression, alpha-fetoprotein (AFP), serum ANGPTL 3 levels, Child-Pugh grade were significantly assosciatedassociated with increased risk of HCC. Logistic regression analysis revealed that ANGPTL 3 expression and AFP levels were the only pridectorspredictors of HCC (odd's ratio (OR)=8.9; 8.6 respectively, P=0.003). Receiver operator characteristic (ROC) demonsterated that serum ANGPTL3 and ANGPTL4 levels were usufuluseful biomarkers discriminating chronic hepatitis cases from controls (AUC=0.820,0.887, respectively P<0.001). However, they fail to discriminate HCC patients from chronic hepatitis patients (P=0.27,0.12 respectively). Moreover, ANGPTL3 and ANGPTL 4 expression were promising biomarkers discriminating chronic hepatitis cases from controls and those HCC cases from chronic hepatitis patients (P<0.001). Combined ANGPTL3 expression and serum level wasn't useful in discriminating HCC patient from chronic hepatitis (P=0.09). In contrast, combined ANGPTL4 expression and serum level was an useful biomarker discriminating HCC cases from chronic hepatitis. CONCLUSION: ANGPTL3 and ANGPTL 4 expression and serum levels can be promising non invasive biomarkers in diagnosis of chronic hepatitis and HCC especially their expression could be useful in discriminating HCC from chronic hepatitis patients.
Synthesis and study of anti-HIV-1 RT activity of 5-benzoyl-4-methyl-1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-one derivatives.[Pubmed:28371664]
Bioorg Chem. 2017 Jun;72:74-79.
In the present study, a series of fourteen 5-benzoyl-4-methyl-1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-one derivatives were designed, synthesized and characterized by appropriate spectral analysis. Further, titled compounds were in-vitro screened against wild HIV-1 RT enzyme using ELISA based colorimetric assay, in which four compounds significantly inhibited the RT activity with IC50
Analysis of Packet-Loss-Induced Distortion in View Synthesis Prediction-Based 3D Video Coding.[Pubmed:28371778]
IEEE Trans Image Process. 2017 Jun;26(6):2781-2796.
View synthesis prediction (VSP) is a crucial coding tool for improving compression efficiency in the next generation 3D video systems. However, VSP is susceptible to catastrophic error propagation when multi-view video plus depth (MVD) data are transmitted over lossy networks. This paper aims at accurately modeling the transmission errors propagated in the inter-view direction caused by VSP. Toward this end, we first study how channel errors gradually propagate along the VSP-based inter-view prediction path. Then, a new recursive model is formulated to estimate the expected end-to-end distortion caused by those channel losses. For the proposed model, the compound impact of the transmission distortions of both the texture video and depth map on the quality of the synthetic reference view is mathematically analyzed. Especially, the expected view synthesis distortion due to depth errors is characterized in the frequency domain using a new approach, which combines the energy densities of the reconstructed texture image and the channel errors. The proposed model also explicitly considers the disparity rounding operation invoked for the sub-pixel precision rendering of the synthesized reference view. Experimental results are presented to demonstrate that the proposed analytic model is capable of effectively modeling the channel-induced distortion for MVD-based 3D video transmission.


