m-Anisic acidCAS# 586-38-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 586-38-9 | SDF | Download SDF |
| PubChem ID | 11461 | Appearance | Powder |
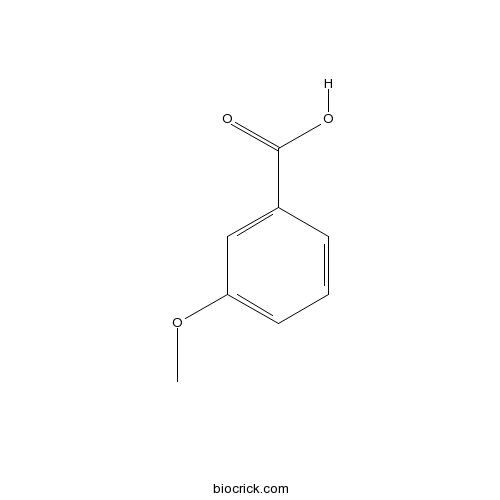
| Formula | C8H8O3 | M.Wt | 152 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-methoxybenzoic acid | ||
| SMILES | COC1=CC=CC(=C1)C(=O)O | ||
| Standard InChIKey | XHQZJYCNDZAGLW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O3/c1-11-7-4-2-3-6(5-7)8(9)10/h2-5H,1H3,(H,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

m-Anisic acid Dilution Calculator

m-Anisic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.5789 mL | 32.8947 mL | 65.7895 mL | 131.5789 mL | 164.4737 mL |
| 5 mM | 1.3158 mL | 6.5789 mL | 13.1579 mL | 26.3158 mL | 32.8947 mL |
| 10 mM | 0.6579 mL | 3.2895 mL | 6.5789 mL | 13.1579 mL | 16.4474 mL |
| 50 mM | 0.1316 mL | 0.6579 mL | 1.3158 mL | 2.6316 mL | 3.2895 mL |
| 100 mM | 0.0658 mL | 0.3289 mL | 0.6579 mL | 1.3158 mL | 1.6447 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anagrelide HCl
Catalog No.:BCC2306
CAS No.:58579-51-4
- Saikosaponin B4
Catalog No.:BCN8516
CAS No.:58558-09-1
- Saikosaponin B1
Catalog No.:BCN5917
CAS No.:58558-08-0
- Losmapimod
Catalog No.:BCC5368
CAS No.:585543-15-3
- Confluentin
Catalog No.:BCN5795
CAS No.:585534-03-8
- Schisantherin A
Catalog No.:BCN1024
CAS No.:58546-56-8
- Schisantherin B
Catalog No.:BCN1023
CAS No.:58546-55-7
- Gomisin A
Catalog No.:BCN5794
CAS No.:58546-54-6
- Cucurbitacin IIA
Catalog No.:BCN5019
CAS No.:58546-34-2
- Rebaudioside B
Catalog No.:BCN2612
CAS No.:58543-17-2
- Rebaudioside A
Catalog No.:BCN5900
CAS No.:58543-16-1
- (-)-Cephaeline dihydrochloride
Catalog No.:BCN8323
CAS No.:5853-29-2
- H- ß-HoGlu-OH.HCl
Catalog No.:BCC3232
CAS No.:58610-41-6
- Boc-ON
Catalog No.:BCC2797
CAS No.:58632-95-4
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- Boc-Cys(Acm)-ONp
Catalog No.:BCC3375
CAS No.:58651-76-6
- 2-C-Methyl-D-erythritol
Catalog No.:BCC8570
CAS No.:58698-37-6
- 3,4-Dichloro-Phe-OH
Catalog No.:BCC2636
CAS No.:587-56-4
- Dihydrokavain
Catalog No.:BCN2677
CAS No.:587-63-3
- H-D-Cha-OH
Catalog No.:BCC2662
CAS No.:58717-02-5
- 16-Oxoprometaphanine
Catalog No.:BCN5797
CAS No.:58738-31-1
- H-D-Ser-OMe.HCl
Catalog No.:BCC3098
CAS No.:5874-57-7
- Licochalcone A
Catalog No.:BCN6332
CAS No.:58749-22-7
- Licochalcone B
Catalog No.:BCN6333
CAS No.:58749-23-8
Treatment of 3,3'-dimethoxybenzidine in sludge by advance oxidation process: Degradation products and toxicity evaluation.[Pubmed:30849594]
J Environ Manage. 2019 May 15;238:102-109.
Studies on the oxidation products of organic pollutants and their toxicity in textile dyeing sludge after the sludge was treated by the advance oxidation processes were limited, since textile dyeing sludge was a complicated mixture. For the first time, simulated sludge was used to study the degradation mechanism of 3,3'-dimethoxybenzidine (DMB) during the combined ultrasound-Mn(VII) treatment. The toxicity of DMB and its products was also evaluated. The results indicated that the compositions and microstructures of polyaluminium chloride (PAC)- and polyferric sulphate (PFS)-based simulated sludge were similar to those of real textile dyeing sludge. The optimum conditions of ultrasound-Mn(VII) treatment were: a KMnO4 dosage of 40muM, an ultrasound power density of 0.36Wcm(-3), and a reaction time of 20min. 98.24% of DMB and 63.04% of total organic carbon (TOC) in the simulated sludge were removed. Six products, that is, 2-nitroanisole, 3-methoxy-4-nitrophenol, vanillylmandelic acid, vanillyl alcohol, m-Anisic acid, and benzoic acid, were identified by GC-MS and LC-MS-MS. It was noted that all of these identified products were also detected in the real textile dyeing sludge after the ultrasound-Mn(VII) treatment. All of them were less toxic than DMB. Moreover, 53.30% and 54.80% of toxicity toward the alga Desmodesmus subspicatus and the bacterium Vibrio fischeri were removed in simulated sludge, respectively. Therefore, simulated sludge was helpful for studying a pollutant's degradation mechanism in the complex sludge mixtures. The results would also provide some useful suggestions for the sludge disposal after the sludge was treated by the advance oxidation processes.


