Rebaudioside BCAS# 58543-17-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58543-17-2 | SDF | Download SDF |
| PubChem ID | 3085143 | Appearance | White powder |
| Formula | C38H60O18 | M.Wt | 804.87 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Stevioside a4 | ||
| Solubility | Soluble in methan | ||
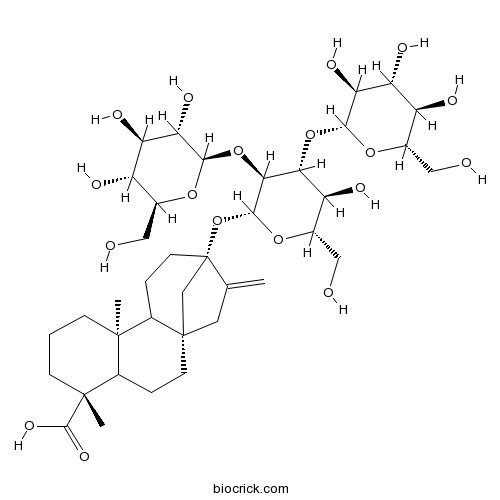
| SMILES | CC12CCCC(C1CCC34C2CCC(C3)(C(=C)C4)OC5C(C(C(C(O5)CO)O)OC6C(C(C(C(O6)CO)O)O)O)OC7C(C(C(C(O7)CO)O)O)O)(C)C(=O)O | ||
| Standard InChIKey | DRSKVOAJKLUMCL-FIPBZOTESA-N | ||
| Standard InChI | InChI=1S/C38H60O18/c1-16-11-37-9-5-20-35(2,7-4-8-36(20,3)34(49)50)21(37)6-10-38(16,15-37)56-33-30(55-32-28(48)26(46)23(43)18(13-40)52-32)29(24(44)19(14-41)53-33)54-31-27(47)25(45)22(42)17(12-39)51-31/h17-33,39-48H,1,4-15H2,2-3H3,(H,49,50)/t17-,18-,19-,20?,21?,22-,23-,24-,25+,26+,27-,28-,29+,30-,31+,32+,33+,35+,36+,37+,38-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rebaudioside B tastes about 150 times sweeter than sucrose and it is non-caloric. |
| Structure Identification | Int J Mol Sci. 2012 Nov 16;13(11):15126-36.Catalytic hydrogenation of the sweet principles of Stevia rebaudiana, Rebaudioside B, Rebaudioside C, and Rebaudioside D and sensory evaluation of their reduced derivatives.[Pubmed: 23203115]Catalytic hydrogenation of Rebaudioside B, rebaudioside C, and rebaudioside D; the three ent-kaurane diterpene glycosides isolated from Stevia rebaudiana was carried out using Pd(OH)(2). Reduction of steviol glycosides was performed using straightforward synthetic chemistry with the catalyst Pd(OH)(2) and structures of the corresponding dihydro derivatives were characterized on the basis of 1D and 2D nuclear magnetic resonance (NMR) spectral data indicating that all are novel compounds being reported for the first time. Also, the taste properties of all reduced compounds were evaluated against their corresponding original steviol glycosides and sucrose. |

Rebaudioside B Dilution Calculator

Rebaudioside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2424 mL | 6.2122 mL | 12.4244 mL | 24.8487 mL | 31.0609 mL |
| 5 mM | 0.2485 mL | 1.2424 mL | 2.4849 mL | 4.9697 mL | 6.2122 mL |
| 10 mM | 0.1242 mL | 0.6212 mL | 1.2424 mL | 2.4849 mL | 3.1061 mL |
| 50 mM | 0.0248 mL | 0.1242 mL | 0.2485 mL | 0.497 mL | 0.6212 mL |
| 100 mM | 0.0124 mL | 0.0621 mL | 0.1242 mL | 0.2485 mL | 0.3106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rebaudioside A
Catalog No.:BCN5900
CAS No.:58543-16-1
- (-)-Cephaeline dihydrochloride
Catalog No.:BCN8323
CAS No.:5853-29-2
- Pseurotin A
Catalog No.:BCN7246
CAS No.:58523-30-1
- IOX 1
Catalog No.:BCC6192
CAS No.:5852-78-8
- ent-16beta,17-Isopropylidenedioxykaurane
Catalog No.:BCN1408
CAS No.:58493-71-3
- Olvanil
Catalog No.:BCC6855
CAS No.:58493-49-5
- Lemannine
Catalog No.:BCN3742
CAS No.:58480-54-9
- Platycodin D
Catalog No.:BCN4982
CAS No.:58479-68-8
- Ferrugine
Catalog No.:BCN1910
CAS No.:58471-11-7
- Darlingine
Catalog No.:BCN1906
CAS No.:58471-10-6
- N-(2-Hydroxy-4-methoxyphenyl)acetamide
Catalog No.:BCN1409
CAS No.:58469-06-0
- H-D-Leu-OMe.HCl
Catalog No.:BCC2681
CAS No.:5845-53-4
- Cucurbitacin IIA
Catalog No.:BCN5019
CAS No.:58546-34-2
- Gomisin A
Catalog No.:BCN5794
CAS No.:58546-54-6
- Schisantherin B
Catalog No.:BCN1023
CAS No.:58546-55-7
- Schisantherin A
Catalog No.:BCN1024
CAS No.:58546-56-8
- Confluentin
Catalog No.:BCN5795
CAS No.:585534-03-8
- Losmapimod
Catalog No.:BCC5368
CAS No.:585543-15-3
- Saikosaponin B1
Catalog No.:BCN5917
CAS No.:58558-08-0
- Saikosaponin B4
Catalog No.:BCN8516
CAS No.:58558-09-1
- Anagrelide HCl
Catalog No.:BCC2306
CAS No.:58579-51-4
- m-Anisic acid
Catalog No.:BCC9015
CAS No.:586-38-9
- H- ß-HoGlu-OH.HCl
Catalog No.:BCC3232
CAS No.:58610-41-6
- Boc-ON
Catalog No.:BCC2797
CAS No.:58632-95-4
Catalytic hydrogenation of the sweet principles of Stevia rebaudiana, Rebaudioside B, Rebaudioside C, and Rebaudioside D and sensory evaluation of their reduced derivatives.[Pubmed:23203115]
Int J Mol Sci. 2012 Nov 16;13(11):15126-36.
Catalytic hydrogenation of Rebaudioside B, rebaudioside C, and rebaudioside D; the three ent-kaurane diterpene glycosides isolated from Stevia rebaudiana was carried out using Pd(OH)(2). Reduction of steviol glycosides was performed using straightforward synthetic chemistry with the catalyst Pd(OH)(2) and structures of the corresponding dihydro derivatives were characterized on the basis of 1D and 2D nuclear magnetic resonance (NMR) spectral data indicating that all are novel compounds being reported for the first time. Also, the taste properties of all reduced compounds were evaluated against their corresponding original steviol glycosides and sucrose.


