ConfluentinCAS# 585534-03-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 585534-03-8 | SDF | Download SDF |
| PubChem ID | 10381750 | Appearance | Oil |
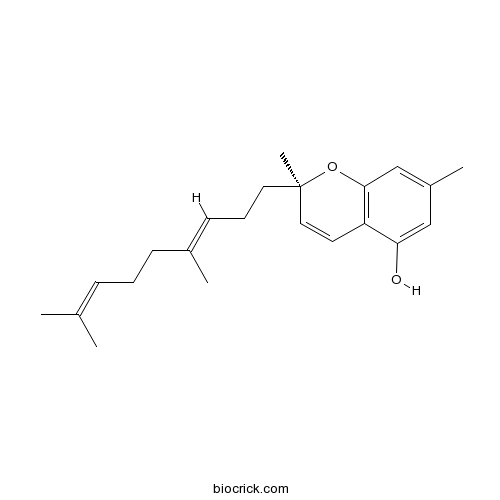
| Formula | C22H30O2 | M.Wt | 326.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R)-2-[(3E)-4,8-dimethylnona-3,7-dienyl]-2,7-dimethylchromen-5-ol | ||
| SMILES | CC1=CC2=C(C=CC(O2)(C)CCC=C(C)CCC=C(C)C)C(=C1)O | ||
| Standard InChIKey | WQOSNKWCIQZRGH-IHSQGBLNSA-N | ||
| Standard InChI | InChI=1S/C22H30O2/c1-16(2)8-6-9-17(3)10-7-12-22(5)13-11-19-20(23)14-18(4)15-21(19)24-22/h8,10-11,13-15,23H,6-7,9,12H2,1-5H3/b17-10+/t22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Confluentin has antimicrobial activity against the gram-positive bacteria. 2. Confluentin significantly inhibits compound 48/80-induced histamine release from rat peritoneal mast cells. 3. Confluentin shows weak cytotoxicity against four human tumor cell lines, HL-60, SMMC-7712, A-549, and MCF-7, in vitro. |
| Targets | Histamine Receptor | Antifection |

Confluentin Dilution Calculator

Confluentin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0628 mL | 15.3139 mL | 30.6279 mL | 61.2557 mL | 76.5697 mL |
| 5 mM | 0.6126 mL | 3.0628 mL | 6.1256 mL | 12.2511 mL | 15.3139 mL |
| 10 mM | 0.3063 mL | 1.5314 mL | 3.0628 mL | 6.1256 mL | 7.657 mL |
| 50 mM | 0.0613 mL | 0.3063 mL | 0.6126 mL | 1.2251 mL | 1.5314 mL |
| 100 mM | 0.0306 mL | 0.1531 mL | 0.3063 mL | 0.6126 mL | 0.7657 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Schisantherin A
Catalog No.:BCN1024
CAS No.:58546-56-8
- Schisantherin B
Catalog No.:BCN1023
CAS No.:58546-55-7
- Gomisin A
Catalog No.:BCN5794
CAS No.:58546-54-6
- Cucurbitacin IIA
Catalog No.:BCN5019
CAS No.:58546-34-2
- Rebaudioside B
Catalog No.:BCN2612
CAS No.:58543-17-2
- Rebaudioside A
Catalog No.:BCN5900
CAS No.:58543-16-1
- (-)-Cephaeline dihydrochloride
Catalog No.:BCN8323
CAS No.:5853-29-2
- Pseurotin A
Catalog No.:BCN7246
CAS No.:58523-30-1
- IOX 1
Catalog No.:BCC6192
CAS No.:5852-78-8
- ent-16beta,17-Isopropylidenedioxykaurane
Catalog No.:BCN1408
CAS No.:58493-71-3
- Olvanil
Catalog No.:BCC6855
CAS No.:58493-49-5
- Lemannine
Catalog No.:BCN3742
CAS No.:58480-54-9
- Losmapimod
Catalog No.:BCC5368
CAS No.:585543-15-3
- Saikosaponin B1
Catalog No.:BCN5917
CAS No.:58558-08-0
- Saikosaponin B4
Catalog No.:BCN8516
CAS No.:58558-09-1
- Anagrelide HCl
Catalog No.:BCC2306
CAS No.:58579-51-4
- m-Anisic acid
Catalog No.:BCC9015
CAS No.:586-38-9
- H- ß-HoGlu-OH.HCl
Catalog No.:BCC3232
CAS No.:58610-41-6
- Boc-ON
Catalog No.:BCC2797
CAS No.:58632-95-4
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- Boc-Cys(Acm)-ONp
Catalog No.:BCC3375
CAS No.:58651-76-6
- 2-C-Methyl-D-erythritol
Catalog No.:BCC8570
CAS No.:58698-37-6
- 3,4-Dichloro-Phe-OH
Catalog No.:BCC2636
CAS No.:587-56-4
- Dihydrokavain
Catalog No.:BCN2677
CAS No.:587-63-3
Antibacterial compounds from mushrooms I: a lanostane-type triterpene and prenylphenol derivatives from Jahnoporus hirtus and Albatrellus flettii and their activities against Bacillus cereus and Enterococcus faecalis.[Pubmed:19644795]
Planta Med. 2010 Feb;76(2):182-5.
Antibacterial bioassay-guided fractionation of two American mushroom species, Jahnoporus hirtus and Albatrellus flettii, led to the isolation and identification of their major antibacterial constituents: 3,11-dioxolanosta-8,24( Z)-diene-26-oic acid (1) from J. hirtus and Confluentin (2), grifolin (3), and neogrifolin (4) from A. flettii. Compound 1 is a new lanostane-type triterpene. All purified compounds were evaluated for their ability to inhibit the growth of Bacillus cereus and Enterococcus faecalis using standard MIC assays. Compounds 1- 4 demonstrated MIC values of 40, 20, 10, and 20 microg/mL, respectively, against B. cereus and MIC values of 32, 1.0, 0.5, and 0.5 microg/mL, respectively, against E. faecalis. Thus, one novel compound and three others were shown to possess antimicrobial activities against these gram-positive bacteria employed as surrogates for more virulent and dangerous pathogens.
Activities of prenylphenol derivatives from fruitbodies of Albatrellus spp. on the human and rat vanilloid receptor 1 (VR1) and characterisation of the novel natural product, confluentin.[Pubmed:12761765]
Arch Pharm (Weinheim). 2003 Apr;336(2):119-26.
Several prenylphenols from basidiocarps of European and Chinese Albatrellus spp., namely grifolin (1), neogrifolin (2), Confluentin (3), scutigeral (4), and albaconol (5) were investigated concerning their activities in test models for vanilloid receptor modulation. The isolation of these compounds from A. confluens and structure elucidation of the novel natural product Confluentin (3) are described. The effects of scutigeral and neogrifolin on vanilloid receptors were studied by means of electrophysiological methodology on rat dorsal root ganglion neurons as well as on recombinant cell lines expressing the rat VR1 receptor. Concurrently, the effects of compounds 1-5 on a reporter cell line expressing the human vanilloid receptor VR1 were measured. In contrast to previous studies reported in the literature, the results of these investigations suggest that fungal prenylphenols act as weak antagonists (activity in the microM range), rather than exhibiting agonistic activities.
Structures and histamine release inhibitory effects of prenylated orcinol derivatives from Rhododendron dauricum.[Pubmed:15270561]
J Nat Prod. 2004 Jul;67(7):1106-9.
Four new prenylated orcinol derivatives, daurichromenes A-D (1-4), along with three known compounds, Confluentin (5), grifolin (6), and orcinol (7), have been isolated from the Chinese medicinal plant Rhododendron dauricum. Their structures were established as 2R-(7'-hydroxy-4',8'-dimethyl-3'E,8'-nonadienyl)-5-hydroxy-2,7-dimethyl-2H-chrome ne (1), 2R-(3'-hydroxy-8'-methyl-4'-methyliden-7'-nonaenyl)-5-hydroxy-2,7-dimethyl-2H-chr omene (2), 2R-(8'-hydroxy-4',8'-dimethyl-3'E,6'Z-nonadienyl)-5-hydroxy-2,7-dimethyl-2H-chrom ene (3), and 2R-(9'-hydroxy-4',8'-dimethyl-3'E,7'E-nonadienyl)-5-hydroxy-2,7-dimethyl-2H-chrom ene (4) by analysis of spectral data. The absolute configuration of the asymmetric carbons at the chromene ring in 1-5 was determined as R from their circular dichroism spectra. Compounds 1-6 significantly inhibited compound 48/80-induced histamine release from rat peritoneal mast cells.
Meroterpenoid pigments from the basidiomycete Albatrellus ovinus.[Pubmed:23305465]
J Nat Prod. 2013 Jan 25;76(1):79-84.
Eight grifolin derivatives, involving three new monomers, albatrelins A-C (1-3), three novel dimers (meroterpenoid pigments), albatrelins D-F (4-6), and two known ones, 6a,7,8,9,10,10a-hexahydro-3,6,9-trimethyl-6-(4-methyl-3-penten-1-yl)-1,9-epoxy-6H -dibenzo[b,d]pyran (7) and Confluentin (8), were isolated from Albatrellus ovinus. Their structures were established by extensive spectroscopic analysis. The absolute configurations of compounds 2-4 were determined as 9R by comparing their optical rotations with data reported in the literature. Albatrelin F (6) was isolated as a pair of C-2' tautomers with a ratio of 1.3:1. Confluentin (8) showed weak cytotoxicity against four human tumor cell lines, HL-60, SMMC-7712, A-549, and MCF-7, in vitro.


