Meranzin hydrateCAS# 5875-49-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5875-49-0 | SDF | Download SDF |
| PubChem ID | 821434 | Appearance | Powder |
| Formula | C15H18O5 | M.Wt | 278.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
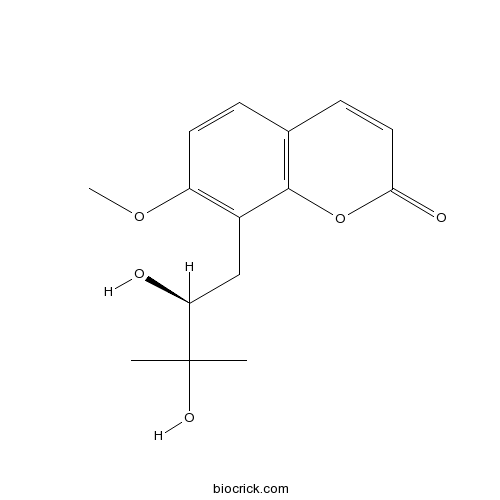
| Chemical Name | 8-[(2S)-2,3-dihydroxy-3-methylbutyl]-7-methoxychromen-2-one | ||
| SMILES | CC(C)(C(CC1=C(C=CC2=C1OC(=O)C=C2)OC)O)O | ||
| Standard InChIKey | KGGUASRIGLRPAX-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C15H18O5/c1-15(2,18)12(16)8-10-11(19-3)6-4-9-5-7-13(17)20-14(9)10/h4-7,12,16,18H,8H2,1-3H3/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Meranzin hydrate produces a rapid effect mediated by AMPA receptors and involving BDNF modulation through the ERK1/2 pathway. 2. Chronic mild stress accelerates the absorption of Meranzin hydrate in rats following the oral administration of Chaihu-Shugan-San. 3. Meranzin hydrate can induce similar effect to Fructus Aurantii on intestinal motility and it was, at least in part, mediated by stimulation of H1 histamine receptors. 4. Meranzin hydrate exhibits antidepressive and prokinetic-like effects through the regulation of the common mediator, the alpha 2-adrenoceptor , and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. |
| Targets | Adrenergic Receptor | ERK |

Meranzin hydrate Dilution Calculator

Meranzin hydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5932 mL | 17.9662 mL | 35.9324 mL | 71.8649 mL | 89.8311 mL |
| 5 mM | 0.7186 mL | 3.5932 mL | 7.1865 mL | 14.373 mL | 17.9662 mL |
| 10 mM | 0.3593 mL | 1.7966 mL | 3.5932 mL | 7.1865 mL | 8.9831 mL |
| 50 mM | 0.0719 mL | 0.3593 mL | 0.7186 mL | 1.4373 mL | 1.7966 mL |
| 100 mM | 0.0359 mL | 0.1797 mL | 0.3593 mL | 0.7186 mL | 0.8983 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Proparacaine HCl
Catalog No.:BCC5073
CAS No.:5875-06-9
- Licochalcone B
Catalog No.:BCN6333
CAS No.:58749-23-8
- Licochalcone A
Catalog No.:BCN6332
CAS No.:58749-22-7
- H-D-Ser-OMe.HCl
Catalog No.:BCC3098
CAS No.:5874-57-7
- 16-Oxoprometaphanine
Catalog No.:BCN5797
CAS No.:58738-31-1
- H-D-Cha-OH
Catalog No.:BCC2662
CAS No.:58717-02-5
- Dihydrokavain
Catalog No.:BCN2677
CAS No.:587-63-3
- 3,4-Dichloro-Phe-OH
Catalog No.:BCC2636
CAS No.:587-56-4
- 2-C-Methyl-D-erythritol
Catalog No.:BCC8570
CAS No.:58698-37-6
- Boc-Cys(Acm)-ONp
Catalog No.:BCC3375
CAS No.:58651-76-6
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- Boc-ON
Catalog No.:BCC2797
CAS No.:58632-95-4
- Haplopine
Catalog No.:BCN3921
CAS No.:5876-17-5
- Pinostilbenoside
Catalog No.:BCN5799
CAS No.:58762-96-2
- Alpha-Belladonnine
Catalog No.:BCN1894
CAS No.:5878-33-1
- ZCL278
Catalog No.:BCC3665
CAS No.:587841-73-4
- C7280948
Catalog No.:BCC6443
CAS No.:587850-67-7
- ABT 724 trihydrochloride
Catalog No.:BCC7293
CAS No.:587870-77-7
- KU 55933
Catalog No.:BCC2475
CAS No.:587871-26-9
- Benzalazine
Catalog No.:BCC8843
CAS No.:588-68-1
- Toosendanin
Catalog No.:BCN1007
CAS No.:58812-37-6
- SB 297006
Catalog No.:BCC6129
CAS No.:58816-69-6
- [Leu5]-Enkephalin
Catalog No.:BCC5831
CAS No.:58822-25-6
- Secoxyloganin
Catalog No.:BCN5800
CAS No.:58822-47-2
The involvement of AMPA-ERK1/2-BDNF pathway in the mechanism of new antidepressant action of prokinetic meranzin hydrate.[Pubmed:22782214]
Amino Acids. 2013 Feb;44(2):413-22.
It was recently discovered that ketamine can relieve depression in a matter of hours through an action on alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. This is much more rapid than the several weeks required for the available antidepressants to show therapeutic efficacy. However, ketamine has negative side effects. The aim of this study was to determine whether the natural prokinetic drug Meranzin hydrate (MH) has a fast-acting antidepressant effect mediated by AMPA receptors. By means of in vivo and in vitro experiments, we found that (1) treatment of rats with MH at 9 mg/kg decreased immobility time in a forced swimming test (FST), as did the popular antidepressant fluoxetine and the AMPA receptor positive modulator aniracetam. Pretreatment of rats with NBQX (10 mg/kg), an antagonist of AMPA receptors, blocked this effect of MH. (2) MH increased number of crossings of forced swimming rats in the open field test. (3) FST enhanced hippocampal ERK1/2, p-ERK1/2 and BDNF expression levels. MH (9 mg/kg) treatment further up-regulated hippocampal p-ERK1/2 and BDNF expression levels, and this effect was prevented by NBQX. (4) MH-increased BDNF expression corresponded with MH-decreased immobility time in the FST. (5) In vitro experiments, we found that incubation of rats hippocampus slices with MH (10, 20 muM respectively) increased concentrations of BDNF and p-ERK1/2. This effect of MH (20 muM) were prevented by NBQX. In conclusion, in animals subjected to acute stress, the natural prokinetic drug MH produced a rapid effect mediated by AMPA receptors and involving BDNF modulation through the ERK1/2 pathway.
Pharmacokinetic study of the prokinetic compounds meranzin hydrate and ferulic acid following oral administration of Chaihu-Shugan-San to patients with functional dyspepsia.[Pubmed:21605652]
J Ethnopharmacol. 2011 Sep 1;137(1):205-13.
AIM OF THE STUDY: The prokinetic activity of ferulic acid derived from Ligusticum chuanxiong hort in the Chaihu-Shugan-San formula has been shown to be similar to Chaihu-Shugan-San, a popular traditional Chinese medicine for treating functional dyspepsia. The effects of Meranzin hydrate, a compound isolated from Fructus aurantii in the Chaihu-Shugan-San formula, are unclear, as the pharmacokinetics have never been studied in patients with functional dyspepsia. This study aimed to describe the pharmacokinetics of ferulic acid and merazin hydrate by evaluating the prokinetics induced by Chaihu-Shugan-San and Meranzin hydrate. MATERIALS AND METHODS: Gastric emptying and intestinal transit were measured after oral administration of a single dose of Chaihu-Shugan-San or Meranzin hydrate in rats. The tone of rat ileum was selected as direct evidence of the prokinetic activity of Meranzin hydrate. Patients with functional dyspepsia were recruited, and Meranzin hydrate and ferulic acid were identified by ultra performance liquid chromatography with tandem mass spectrometry in the plasma of patients following a single oral administration of Chaihu-Shugan-San. The resulting pharmacokinetic properties were determined by ultra performance liquid chromatography coupled to photo diode array. RESULTS: In rats, single doses of Chaihu-Shugan-San (20 g/kg) and Meranzin hydrate (28 mg/kg) significantly accelerated gastric emptying and intestinal transit (Chaihu-Shugan-San: 68.9 +/- 5.6% and 72.3 +/- 4.7%, Meranzin hydrate: 72.9 +/- 3.8% and 75.2 +/- 3.1%) compared with the control (55.45 +/- 3.7% and 63.51 +/- 5.1%, P<0.05), showing similar results as cisapride (69.6 +/- 4.8% and 71.6 +/- 6.3%). Meranzin hydrate (30, 100 mumol/L) directly increased the amplitude of rat ileum compared with the control (P<0.01). The pharmacokinetics profiles of Meranzin hydrate and ferulic acid in patient plasma was fitted with a two-compartment model detected by a simple, rapid and accurate UPLC method. Time to reach peak concentration of Meranzin hydrate (0.371 mg/L) and ferulic acid (0.199 mg/L) was 23.57 min and 27.50 min, respectively. The elimination half-life and area under the concentration-time curve from t=0 to the last time of Meranzin hydrate and ferulic acid were 139.53 min and 31.445 mug min/mL and 131.27 min and 14.835 mug min/mL, respectively. The absorption constant and volume of distribution of Meranzin hydrate and ferulic acid were 0.185 +/- 0.065 min(-1) and 3782.89 +/- 2686.72 L/kg and 0.524 +/- 0.157 min(-1) and 11713 +/- 7618.68 L/kg, respectively. The experimental results of the pharmacokinetic parameters of Meranzin hydrate and ferulic acid indicate that they were absorbed and distributed rapidly. CONCLUSIONS: The pharmacodynamics and pharmacokinetics of prokinetic Chaihu-Shugan-San and its compounds are useful for monitoring Chaihu-Shugan-San formulas in clinical practice and for understanding therapeutic mechanisms.
Meranzin hydrate induces similar effect to Fructus Aurantii on intestinal motility through activation of H1 histamine receptors.[Pubmed:21061180]
J Gastrointest Surg. 2011 Jan;15(1):87-96.
This experiment studied the potential effect of Meranzin hydrate (MH) and decoction of herb Fructus Aurantii (FA) on rat gut motility. It also investigated the prokinetic mechanism of MH. Experiments were performed on male Sprague-Dawley rats (200-220 g). The study included: (1) qualitation of MH and four other known compounds in FA and jejunum after oral administration of FA decoction to rats; (2) in vitro experiment of MH on rat jejunum contractions; (3) in vivo experiment of FA and MH in rats. Dose-dependently, MH (1-100 muM) increased amplitude in longitudinal and circular jejunum muscles. Pretreatment of jejunum longitudinal strips with benzhydramine (1 muM) remarkably inhibited the contractions induced by histamine (1 muM) and MH (10 or 30 muM). Pretreatment of jejunum longitudinal strips with atropine (1 muM) reduced the contractions induced by acetylcholine (1 muM) but did not influence the contractions induced by MH (10 or 30 muM). Interestingly, the antagonism of benzhydramine to MH was also verified in vivo. MH can be absorbed into the jejunum following oral administration of FA decoction. In healthy rats, MH (7, 14, and 28 mg/kg) and FA (3.3, 10, and 20 g/kg) both promoted intestinal transit and gastric emptying in a dose-dependent manner when gavaged acutely. In cisplatin model rats, MH (14 and 28 mg/kg) significantly reversed cisplatin-induced delay in gastric emptying. Meranzin hydrate can induce similar effect to Fructus Aurantii on intestinal motility and it was, at least in part, mediated by stimulation of H1 histamine receptors.
Comparison between the pharmacokinetics of meranzin hydrate in a rat model of chronic depression and in controls following the oral administration of Chaihu-Shugan-San.[Pubmed:24137289]
Exp Ther Med. 2013 Oct;6(4):913-918.
Previous studies have shown that Meranzin hydrate (MH) may be beneficial in depressive disorders. However, to the best of our knowledge, the pharmacokinetic characteristics of MH in depression have not previously been investigated. Chronic mild stress (CMS) in rats is used as a model of depression. The present study was designed to evaluate and compare the pharmacokinetics of MH in CMS and control rats following the oral administration of Chaihu-Shugan-San (CSS). Rats were randomly divided into CMS and control groups and blood samples were obtained following the oral administration of CSS. The quantification of MH levels in the plasma for pharmacokinetic study was achieved using a simple and rapid ultra-performance liquid chromatography with photodiode array (UPLC-PDA) method. Following the oral administration of CSS to CMS rats and controls, the maximum plasma concentration (Cmax) of MH was 58.66+/-6.64 and 57.54+/-12.67 ng/ml at 108.00+/-26.83 and 54.00+/-8.22 min, respectively. Compared with the value of the area under the concentration-time curve (AUC)0-1440 in control rats (19,896.76+/-1,041.95 mug.min/l), the AUC0-1440 value was reduced in CMS rats (18,401.32+/-4332.65 mug.min/l). There were no significant differences in the majority of the pharmacokinetic parameters of MH, including the values for Cmax, AUC0-1440, clearance rate (CL/F) and mean residence time (MRT0-1440), between the CMS rats and the controls. However, the pharmacokinetic parameters showed that CMS accelerated the absorption of MH in rats following the oral administration of CSS.
Meranzin hydrate exhibits anti-depressive and prokinetic-like effects through regulation of the shared alpha2-adrenoceptor in the brain-gut axis of rats in the forced swimming test.[Pubmed:23063894]
Neuropharmacology. 2013 Apr;67:318-25.
BACKGROUND: In recent years, the brain-gut axis theory has received increasing attention in studies of depression. However, most studies separately address potential antidepressant and prokinetic treatments. Investigations of drugs that could potentially treat comorbid depression and gastrointestinal (GI) dysfunction via a common mechanism of action have not yet been performed in detail. AIM: To find a common mechanism of action of our patented drug, Meranzin hydrate (MH), in the antidepressant and prokinetic treatment. METHODS: The forced swimming test (FST) model of depression, plasma ghrelin measurement, and in vivo and in vitro measurements of GI motility were used. RESULTS: 1. Administration of MH (9 mg/kg) decreased the immobility time during the FST after acute treatment; this effect was inhibited by the alpha 2-adrenoceptor antagonist, yohimbine, but not by the alpha 1-adrenoceptor antagonist, prazosin. 2. After chronic treatment, the immobility time of rats during the FST was decreased significantly by MH (2.25 mg/kg). 3. MH (9 mg/kg) increased plasma ghrelin levels in rats subjected to the FST; this increase was enhanced by the ghrelin receptor agonist, GHRP-6. 4. MH (9 mg/kg) also promoted gastric emptying and intestinal transit in rats with or without FST. 5. In vitro, MH (10 muM) increased jejunal contractions in rats subjected to the FST; this effect was inhibited by yohimbine. Furthermore, the inhibitory effect of yohimbine was partly reversed by the ghrelin receptor agonist, GHRP-6. CONCLUSION: Our study revealed that MH from natural resources exhibits antidepressive and prokinetic-like effects through the regulation of the common mediator, the alpha 2-adrenoceptor.


