DL-AP4 Sodium saltBroad spectrum EAA antagonist CAS# 1263093-79-3 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Fumonisin B1
Catalog No.:BCC2461
CAS No.:116355-83-0
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1263093-79-3 | SDF | Download SDF |
| PubChem ID | 52974248 | Appearance | Powder |
| Formula | C4H9NNaO5P | M.Wt | 205.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
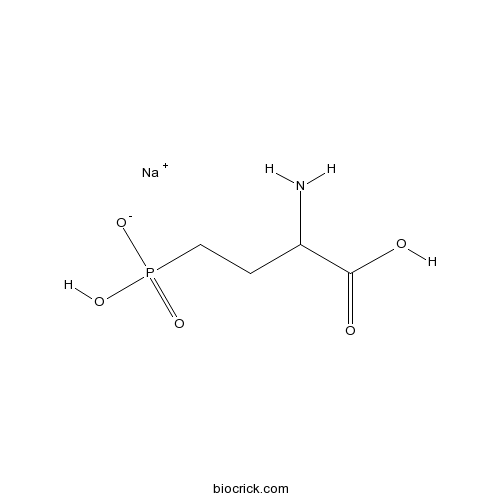
| Chemical Name | sodium;(3-amino-3-carboxypropyl)-hydroxyphosphinate | ||
| SMILES | C(CP(=O)(O)[O-])C(C(=O)O)N.[Na+] | ||
| Standard InChIKey | IGWPQOULJIILQQ-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C4H10NO5P.Na/c5-3(4(6)7)1-2-11(8,9)10;/h3H,1-2,5H2,(H,6,7)(H2,8,9,10);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sodium salt of the broad spectrum EAA ligand DL-AP4. Separate isomers D-AP4 and L-AP4 also available. |

DL-AP4 Sodium salt Dilution Calculator

DL-AP4 Sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8761 mL | 24.3807 mL | 48.7615 mL | 97.5229 mL | 121.9036 mL |
| 5 mM | 0.9752 mL | 4.8761 mL | 9.7523 mL | 19.5046 mL | 24.3807 mL |
| 10 mM | 0.4876 mL | 2.4381 mL | 4.8761 mL | 9.7523 mL | 12.1904 mL |
| 50 mM | 0.0975 mL | 0.4876 mL | 0.9752 mL | 1.9505 mL | 2.4381 mL |
| 100 mM | 0.0488 mL | 0.2438 mL | 0.4876 mL | 0.9752 mL | 1.219 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Paromomycin Sulfate
Catalog No.:BCC4694
CAS No.:1263-89-4
- NCX 466
Catalog No.:BCC6219
CAS No.:1262956-64-8
- Spautin-1
Catalog No.:BCC6420
CAS No.:1262888-28-7
- CCT241533
Catalog No.:BCC1462
CAS No.:1262849-73-9
- 25-Hydroxy VD2-D6
Catalog No.:BCC1305
CAS No.:1262843-46-8
- Trigonosin F
Catalog No.:BCN6403
CAS No.:1262842-73-8
- Penitrem A
Catalog No.:BCC7957
CAS No.:12627-35-9
- Benzoylmesaconine hydrochloride
Catalog No.:BCN5399
CAS No.:126266-38-4
- GS967
Catalog No.:BCC6401
CAS No.:1262618-39-2
- Assamicadine
Catalog No.:BCN1957
CAS No.:126260-96-6
- Fumitremorgin B
Catalog No.:BCN6453
CAS No.:12626-17-4
- (2R,3S)-Dihydrodehydroconiferyl alcohol
Catalog No.:BCN7886
CAS No.:126253-41-6
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- Pinobanksin 3-O-propanoate
Catalog No.:BCN7737
CAS No.:126394-70-5
- Nortropinyl cinnamate
Catalog No.:BCN1891
CAS No.:126394-79-4
- Colistin Sulfate
Catalog No.:BCC4653
CAS No.:1264-72-8
- SR 12813
Catalog No.:BCC7530
CAS No.:126411-39-0
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- Pyrrolam A
Catalog No.:BCN2040
CAS No.:126424-76-8
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- (R)-CPP
Catalog No.:BCC6581
CAS No.:126453-07-4
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations.[Pubmed:7042024]
Br J Pharmacol. 1982 Jan;75(1):65-75.
1 The depressant actions on evoked electrical activity and the excitant amino acid antagonist properties of a range of omega-phosphonic alpha-carboxylic amino acids have been investigated in the isolated spinal cord preparations of the frog or immature rat. 2 When tested on dorsal root-evoked ventral root potentials, members of the homologous series from 2- amino-5-phosphonovaleric acid to 2-amino-8-phosphonooctanoic acid showed depressant actions which correlated with the ability of the substances to antagonize selectivity motoneuronal depolarizations induced by N-methyl-D-aspartate. 3 2-Amino-5-phosphonovalerate was the most potent substance of the series giving an apparent KD of 1.4 microM for the antagonism of responses to N-methyl-D-aspartate. 4 A comparison of the (+)- and (-)-forms of 2-amino-5-phosphonovalerate indicated that the N-methyl-D-aspartate antagonist activity and the neuronal depressant action of this substance were both due mainly to the (-)-isomer. 5 The (-)- and (+)-forms of 2-amino-4-phosphonobutyrate had different actions. The (-)-forms of this substance had a relatively weak and non-selective antagonist action on depolarizations induced by N-methyl-D-aspartate, quisqualate and kainate and a similarly weak depressant effect when tested on evoked electrical activity. The (+)-form was more potent than he (-)-form in depressing electrically evoked activity but did not antagonize responses to amino acid excitants. At concentrations higher than those required to depress electrically evoked activity, the (+)-form produced depolarization. This action was blocked by 2-amino-5-phosphonovalerate.
Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog.[Pubmed:316343]
Br J Pharmacol. 1979 Dec;67(4):591-603.
1. A range of compounds has been tested for excitatory amino acid agonist or antagonist activity and for effects on synaptic activity on isolated hemisected spinal cords of frogs. 2. L-Monoamino dicarboxylic acids of chain length up to 8 carbon atoms (L-alpha-aminosuberate) were all agonists. 3. Within a series of D-monoamino dicarboxylic acids, and with diamino dicarboxylic acids (mainly unresolved mixtures of diasteroisomers), there was a progression from agonist activity, for compounds of chain length equal to or shorter than glutamate, to antagonist activity, for compounds of longer chain length equal to or shorter than glutamate, to antagonist activity, for compounds of longer chain length, D-alpha-Aminosuberate (D alpha SD) was the most potent antagonist. 4. The antagonist actions of these substances showed a Mg2+--like selectivity with respect to depolarizations produced by different excitants. N-methyl-D-aspartate (NMDA) was the most susceptible agonist and quisqualate and kainate the least susceptible. Responses to other excitatory amino acids, including L-glutamate and L-aspartate, showed intermediate sensitivity to the antagonists. 5. A parallelism was observed between the relative potencies of mono- and diamino dicarboxylic acids as NMDA antagonists and their relative potencies as depressants of synaptic responses. 6. The results support the concept of different types of excitatory amino acid receptors, with NMDA and its antagonists acting predominantly on one type. These NMDA receptors are probably transmitter receptors activated by an excitatory amino acid transmitter.


