Fumonisin B1PP5/PP2Cα/PP2A/PP1γ2/PP2B inhibitor CAS# 116355-83-0 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 116355-83-0 | SDF | Download SDF |
| PubChem ID | 62314 | Appearance | Powder |
| Formula | C34H59NO15 | M.Wt | 721.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Methanol : 10 mg/mL (13.85 mM; Need ultrasonic and warming) | ||
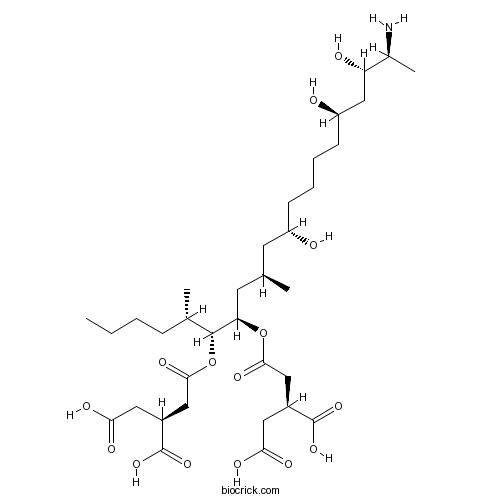
| Chemical Name | (2S)-2-[2-[(5S,6R,7R,9R,11S,16R,18S,19S)-19-amino-6-[(3S)-3,4-dicarboxybutanoyl]oxy-11,16,18-trihydroxy-5,9-dimethylicosan-7-yl]oxy-2-oxoethyl]butanedioic acid | ||
| SMILES | CCCCC(C)C(C(CC(C)CC(CCCCC(CC(C(C)N)O)O)O)OC(=O)CC(CC(=O)O)C(=O)O)OC(=O)CC(CC(=O)O)C(=O)O | ||
| Standard InChIKey | UVBUBMSSQKOIBE-ZWKVXHQASA-N | ||
| Standard InChI | InChI=1S/C34H59NO15/c1-5-6-9-20(3)32(50-31(44)17-23(34(47)48)15-29(41)42)27(49-30(43)16-22(33(45)46)14-28(39)40)13-19(2)12-24(36)10-7-8-11-25(37)18-26(38)21(4)35/h19-27,32,36-38H,5-18,35H2,1-4H3,(H,39,40)(H,41,42)(H,45,46)(H,47,48)/t19-,20+,21+,22+,23+,24+,25-,26+,27-,32-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mycotoxin produced by Fusarium moniliforme. Potently inhibits sphingosine N-acyltransferase (ceramide synthase), causing an accumulation of sphingoid bases (IC50 ~ 75 nM). Also inhibits protein phosphatases; IC50 values are 80, 300, 400, 500 and 3000 μM for PP5, PP2Cα, PP2A, PP1γ2 and PP2B respectively. |

Fumonisin B1 Dilution Calculator

Fumonisin B1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3854 mL | 6.9268 mL | 13.8537 mL | 27.7074 mL | 34.6342 mL |
| 5 mM | 0.2771 mL | 1.3854 mL | 2.7707 mL | 5.5415 mL | 6.9268 mL |
| 10 mM | 0.1385 mL | 0.6927 mL | 1.3854 mL | 2.7707 mL | 3.4634 mL |
| 50 mM | 0.0277 mL | 0.1385 mL | 0.2771 mL | 0.5541 mL | 0.6927 mL |
| 100 mM | 0.0139 mL | 0.0693 mL | 0.1385 mL | 0.2771 mL | 0.3463 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mycotoxin produced by Fusarium moniliforme. Potently inhibits sphingosine N-acyltransferase (ceramide synthase), causing an accumulation of sphingoid bases (IC50 ~ 75 nM). Also inhibits protein phosphatases; IC50 values are 80, 300, 400, 500 and 3000 μM for PP5, PP2Cα, PP2A, PP1γ2 and PP2B respectively.
- SKF 86002 dihydrochloride
Catalog No.:BCC7236
CAS No.:116339-68-5
- Sarafotoxin S6b
Catalog No.:BCC5720
CAS No.:116303-65-2
- Clemizole hydrochloride
Catalog No.:BCC1486
CAS No.:1163-36-6
- 6-Aldehydoisoophiopogonanone A
Catalog No.:BCN2860
CAS No.:116291-82-8
- Pyrroside B
Catalog No.:BCN4042
CAS No.:116271-35-3
- MCB-613
Catalog No.:BCC3982
CAS No.:1162656-22-5
- Levobetaxolol HCl
Catalog No.:BCC4671
CAS No.:116209-55-3
- Aflatoxin B1
Catalog No.:BCC9212
CAS No.:1162-65-8
- Complanatoside
Catalog No.:BCN8213
CAS No.:116183-66-5
- Alexine
Catalog No.:BCN2054
CAS No.:116174-63-1
- Brevicolline
Catalog No.:BCN2459
CAS No.:20069-02-7
- VU 0361737
Catalog No.:BCC4596
CAS No.:1161205-04-4
- Acetylexidonin
Catalog No.:BCN3279
CAS No.:116368-90-2
- 2alpha-hydroxy-3beta-acetyloxy-betulic acid
Catalog No.:BCN3072
CAS No.:1163728-89-9
- Loureirin C
Catalog No.:BCN3761
CAS No.:116384-24-8
- 3',4',7-Trimethoxyflavan
Catalog No.:BCN6042
CAS No.:116384-26-0
- Z-Tyr-OH
Catalog No.:BCC2747
CAS No.:1164-16-5
- Androstanolone acetate
Catalog No.:BCC8826
CAS No.:1164-91-6
- SR 3576
Catalog No.:BCC7999
CAS No.:1164153-22-3
- 9S-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3080
CAS No.:1164201-85-7
- Fargesone A
Catalog No.:BCN6417
CAS No.:116424-69-2
- Fargesone B
Catalog No.:BCN6415
CAS No.:116424-70-5
- Aerugidiol
Catalog No.:BCN3529
CAS No.:116425-35-5
- Curcumadione
Catalog No.:BCN3525
CAS No.:116425-36-6
Oral administration of fumonisin B1 and T-2 individually and in combination affects hepatic total and mitochondrial membrane lipid profile of rabbits.[Pubmed:28229635]
Physiol Int. 2016 Sep;103(3):321-333.
Weaned rabbits were fed diets contaminated with 2 mg/kg diet T-2 toxin alone, or 10 mg/kg diet Fumonisin B1 (FB1) alone, and both toxins in combination (2 + 10 mg/kg, respectively) compared to a toxin-free control diet. Samplings were performed after 4 weeks (blood and liver). Bodyweight of T-2-fed group was lower after 4 weeks; the liver weight was increased dramatically (threefold of control). Liver total phospholipids (PLs) provided slight alterations in the fatty acid (FA) composition; all three toxin-treated groups showed a decrease in palmitoleic acid (C16:1 n7) proportion. In the liver mitochondrial PL FA composition, margaric acid (C17:0) proportion decreased in the separated toxin treatments compared to the combined setting. Oleic acid (C18:1 n9) proportion was increased and arachidonic acid (C20:4 n6) was decreased in the FB1-treated group, while docosapentaenoic acid (C22:5 n3) was decreased in the separated treatments. The total monounsaturation was significantly higher in the FB1 group's mitochondrial PL FA profile. After 4 weeks, all toxin treatments decreased the blood plasma reduced glutathione and glutathione peroxidase activity, and FB1 increased the plasma sphinganine/sphingosine ratio. Both mycotoxins seem to cross the hepatocellular and the hepatic mitochondrial membrane, without drastic membrane disruption, as assessed from the PL FA composition, but inducing detectable lipid peroxidation.
Synthesis and degradation of long-chain base phosphates affect fumonisin B1-induced cell death in Arabidopsis thaliana.[Pubmed:28303405]
J Plant Res. 2017 May;130(3):571-585.
Fumonisin B1 (FB1), an inducer of cell death, disrupts sphingolipid metabolism; large accumulations of de novo synthesized free long-chain bases (LCBs) are observed. However, it remains unclear whether tolerance to FB1 toxicity in plants is connected with preventing the accumulation of free LCBs through their phosphorylation. Here a workflow for the extraction, detection and quantification of LCB phosphates (LCBPs) in Arabidopsis thaliana was developed. We studied the effect of expression of genes for three enzymes involved in the synthesis and degradation of LCBPs, LCB kinase (LCBK1), LCBP phosphatase (SPP1) and lyase (DPL1) on FB1-induced cell death. As expected, large accumulations of saturated free LCBs, dihydrosphingosine and phytosphingosine, were observed in the FB1-treated leaves. On the other hand, a high level of sphingenine phosphate was found in the FB1-treated leaves even though free sphingenine was found in low amounts in these leaves. In comparison of WT and spp1 plants, the LCBP/LCB ratio is likely to be correlated with the degree of FB1-induced cell death determined by trypan blue staining. The FB1-treated leaves in dpl1 plants showed severe cell death and the elevation of free LCBs and LCBPs. LCBK1-OX and -KD plants showed resistance and sensitivity to FB1, respectively, whereas free LCB and LCBP levels in FB1-treated LCBK1-OX and -KD plants were moderately different to those in FB1-treated WT plants. Overall, the findings described here suggest that LCBP/LCB homeostasis is an important topic that participates in the tolerance of plant cells to FB1.
The aflatoxin B1 -fumonisin B1 toxicity in BRL-3A hepatocytes is associated to induction of cytochrome P450 activity and arachidonic acid metabolism.[Pubmed:28181396]
Environ Toxicol. 2017 Jun;32(6):1711-1724.
Human oral exposure to aflatoxin B1 (AFB1 ) and Fumonisin B1 (FB1 ) is associated with increased hepatocellular carcinoma. Although evidence suggested interactive AFB1 -FB1 hepatotoxicity, the underlying mechanisms remain mostly unidentified. This work was aimed at evaluating the possible AFB1 -FB1 interplay to induce genetic and cell cycle toxicities in BRL-3A rat hepatocytes, reactive oxygen species (ROS) involvement, and the AFB1 metabolizing pathways cytochrome P450 (CYP) and arachidonic acid (ArAc) metabolism as ROS contributors. Flow cytometry of stained BRL-3A hepatocytes was used to study the cell cycle (propidium iodide), ROS intracellular production (DCFH-DA, HE, DAF-2 DA), and phospholipase A activity (staining with bis-BODIPY FL C11-PC). The CYP1A activity was assessed by the 7-ethoxyresorufin-O-deethylase (EROD) assay. Despite a 48-h exposure to FB1 (30 muM) not being genotoxic, the AFB1 (20 muM)-induced micronucleus frequency was overcome by the AFB1 -FB1 mixture (MIX), presumably showing toxin interaction. The mycotoxins blocked G1/S-phase, but only MIX caused cell death. Overall, the oxidative stress led these alterations as the pretreatment with N-acetyl-l-cysteine reduced such toxic effects. While AFB1 had a major input to the MIX pro-oxidant activity, with CYP and ArAc metabolism being ROS contributors, these pathways were not involved in the FB1 -elicited weak oxidative stress. The MIX-induced micronucleus frequency in N-acetyl-l-cysteine pretreated cells was greater than that caused by AFB1 without antioxidants, suggesting enhanced AFB1 direct genotoxicity probably owing to the higher CYP activity and ArAc metabolism found in MIX. The metabolic pathways modulation by AFB1 -FB1 mixtures could raise its hepatocarcinogenic properties.
Efficacy of fungal and bacterial antagonists for controlling growth, FUM1 gene expression and fumonisin B1 production by Fusarium verticillioides on maize cobs of different ripening stages.[Pubmed:28213318]
Int J Food Microbiol. 2017 Apr 4;246:72-79.
This study was carried out to examine the efficacy of two biocontrol agents (Clonostachys rosea 016, BCA1; Gram-negative bacterium, BCA5) for control of FUM1 gene expression and Fumonisin B1 (FB1) production by F. verticillioides FV1 on maize cobs of different ripening stages: R3, Milk (0.985 aw); R4, Dough (0.976 aw); R5, Dent (0.958 aw). Initially, temporal studies on FUM1 gene expression and FB1 production were performed on maize kernels for up to 14days. This revealed that day 10 was optimum for both parameters, and was used in the biocontrol studies. Maize cobs were inoculated with 50:50 mixtures of the pathogen:antagonist inoculum and incubated in environmental chambers to maintain the natural aw conditions for ten days at 25 and 30 degrees C. The growth rates of F. verticillioides FV1, the relative expression of the FUM1 gene and FB1 production were quantified. It was found that, awxtemp had significant impacts on growth, FUM1 gene expression and FB1 production by F. verticillioides FV1 on maize cobs of different maturities. The fungal antagonist (BCA1) significantly reduced FB1 contamination on maize cobs by >70% at 25 degrees C, and almost 60% at 30 degrees C regardless of maize ripening stage. For the bacterial antagonist (BCA5) however, FB1 levels on maize cobs were significantly decreased only in some treatments. These results suggest that efficacy of antagonists to control mycotoxin production in ripening maize cobs needs to take account of the ecophysiology of the pathogen and the antagonists, as well as the physiological status of the maize during silking to ensure effective control.
Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression.[Pubmed:18339876]
Cancer Res. 2008 Mar 15;68(6):1945-52.
Cannabinoids, the active components of Cannabis sativa L. and their derivatives, inhibit tumor growth in laboratory animals by inducing apoptosis of tumor cells and impairing tumor angiogenesis. It has also been reported that these compounds inhibit tumor cell spreading, but the molecular targets of this cannabinoid action remain elusive. Here, we evaluated the effect of cannabinoids on matrix metalloproteinase (MMP) expression and its effect on tumor cell invasion. Local administration of Delta(9)-tetrahydrocannabinol (THC), the major active ingredient of cannabis, down-regulated MMP-2 expression in gliomas generated in mice, as determined by Western blot, immunofluorescence, and real-time quantitative PCR analyses. This cannabinoid-induced inhibition of MMP-2 expression in gliomas (a) was MMP-2-selective, as levels of other MMP family members were unaffected; (b) was mimicked by JWH-133, a CB(2) cannabinoid receptor-selective agonist that is devoid of psychoactive side effects; (c) was abrogated by Fumonisin B1, a selective inhibitor of ceramide biosynthesis; and (d) was also evident in two patients with recurrent glioblastoma multiforme. THC inhibited MMP-2 expression and cell invasion in cultured glioma cells. Manipulation of MMP-2 expression by RNA interference and cDNA overexpression experiments proved that down-regulation of this MMP plays a critical role in THC-mediated inhibition of cell invasion. Cannabinoid-induced inhibition of MMP-2 expression and cell invasion was prevented by blocking ceramide biosynthesis and by knocking-down the expression of the stress protein p8. As MMP-2 up-regulation is associated with high progression and poor prognosis of gliomas and many other tumors, MMP-2 down-regulation constitutes a new hallmark of cannabinoid antitumoral activity.
Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ.[Pubmed:8262970]
J Biol Chem. 1993 Dec 25;268(36):27299-306.
Fumonisins, mycotoxins produced by Fusarium moniliforme and a number of other fungi, cause neuronal degeneration, liver and renal toxicity, cancer, and other injury to animals. Recent work with rat hepatocytes (Wang, E., Norred, W. P., Bacon, C. W., Riley, R. T., and Merrill, A. H., Jr. (1991) J. Biol. Chem. 266, 14486-14490) found that fumonisins block sphingosine biosynthesis by inhibiting the conversion of sphinganine to dihydroceramides, which precedes introduction of the 4,5-trans-double bond of sphingosine. The current study utilized mouse cerebellar neurons in culture to evaluate how this affects the distribution of newly synthesized ceramides among different complex sphingolipids. Fumonisin B1 inhibited ceramide synthase in mouse brain microsomes with a competitive-like kinetic behavior with respect to both sphinganine and stearoyl-CoA. Fumonisin B1 inhibited sphingolipid biosynthesis in cultured cerebellar neurons in situ as reflected by accumulation of free sphinganine, a reduction in the mass of total sphingolipids, reductions in the incorporation of [14C]serine into glucosylceramide, lactosylceramide, sphingomyelin, and gangliosides (GM1, GD3, GD1a, GD1b, GT1b, and GQ1b), and inhibition of the incorporation of [14C]galactose and [3H]sphinganine into complex sphingolipids. Dose-response studies revealed that the labeling of sphingomyelin (IC50 of 0.7 microM) was more sensitive to inhibition by Fumonisin B1 than was glycolipid formation (IC50 of approximately 7 microM) in these cells. A similar effect was seen when beta-fluoroalanine was added to inhibit the activity of serine palmitoyltransferase, the first enzyme of the pathway. The inhibition of complex sphingolipid synthesis was reversible, and nearly normal labeling profiles were obtained 48 h after removing the mycotoxin. These studies establish that Fumonisin B1 inhibits de novo sphingolipid biosynthesis by neuronal cells and, moreover, that limiting ceramide synthesis differentially affects the formation of sphingomyelin versus glycosphingolipids.


