MCB-613stimulator of steroid receptor coactivator (SRC) CAS# 1162656-22-5 |
- C646
Catalog No.:BCC4546
CAS No.:328968-36-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1162656-22-5 | SDF | Download SDF |
| PubChem ID | 2175947 | Appearance | Powder |
| Formula | C19H19N3O | M.Wt | 305.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >13.2mg/mL in DMSO | ||
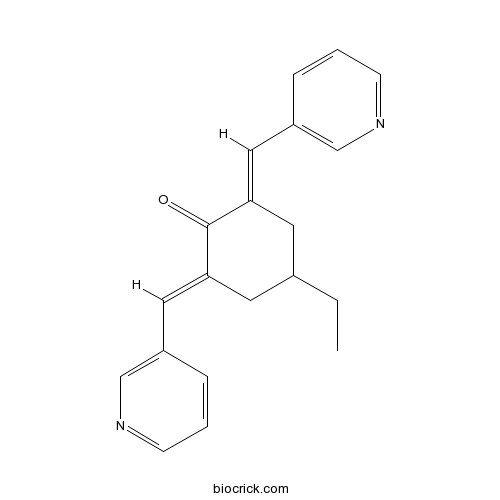
| Chemical Name | (2E,6E)-4-ethyl-2,6-bis(pyridin-3-ylmethylidene)cyclohexan-1-one | ||
| SMILES | CCC1CC(=CC2=CN=CC=C2)C(=O)C(=CC3=CN=CC=C3)C1 | ||
| Standard InChIKey | MMBSCBVEHMETSA-GDAWTGGTSA-N | ||
| Standard InChI | InChI=1S/C20H20N2O/c1-2-15-9-18(11-16-5-3-7-21-13-16)20(23)19(10-15)12-17-6-4-8-22-14-17/h3-8,11-15H,2,9-10H2,1H3/b18-11+,19-12+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MCB-613 is a potent, pan steroid receptor coactivator (SRC) stimulator.
Target: SRC
in vitro: MCB-613 exerts the greatest activation of SRC-1 in the primary screen, is confirmed to be a strong activator of all three SRCs. MCB-613 can super-stimulate SRCs' transcriptional activity. MCB-613 increases SRCs' interactions with other coactivators and markedly induces ER stress coupled to the generation of reactive oxygen species (ROS). MCB-613 selectively and reversibly binds to the RID of SRC-3, and selectively kills cancer cells including MCF-7 (breast), PC-3 (prostate), H1299 (lung), and HepG2 (liver) cells, without toxicity to mouse primary hepatocytes and mouse embryonic fibroblasts (MEFs). MCB-613 also increases SRCs' interactions with other coactivators and markedly induces ER stress coupled to the generation of reactive oxygen species (ROS). [1]
in vivo: In an MCF-7 breast cancer mouse xenograft model, MCB-613 (20 mg/kg, i.p.) significantly and dramatically inhibits the growth of the tumor while causing no obvious animal toxicity and body weight less. [1] References: | |||||

MCB-613 Dilution Calculator

MCB-613 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2747 mL | 16.3736 mL | 32.7472 mL | 65.4943 mL | 81.8679 mL |
| 5 mM | 0.6549 mL | 3.2747 mL | 6.5494 mL | 13.0989 mL | 16.3736 mL |
| 10 mM | 0.3275 mL | 1.6374 mL | 3.2747 mL | 6.5494 mL | 8.1868 mL |
| 50 mM | 0.0655 mL | 0.3275 mL | 0.6549 mL | 1.3099 mL | 1.6374 mL |
| 100 mM | 0.0327 mL | 0.1637 mL | 0.3275 mL | 0.6549 mL | 0.8187 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MCB-613, 4-ethyl-2,6-bis-pyridin-3-ylmethylene-cyclohexanone, is a novel and potent stimulator of p160 steroid receptor coactivators (SRCs). It is a Pan-SRCs stimulator. MCB-613 is confirmed to be a strong activator of all three SRCs family proteins [1].
Members of the SRCs family interact with nuclear receptors and other transcription factors to drive target gene expression while also functioning as integrators of upstream cell signaling pathways. Three factors share homology with each other, they have distinct and important roles in multiple physiological processes, including growth and development, reproduction, and metabolism. All of them have been found to be broadly involved in different tumorigenesis. [2, 3]
MCB-613 can super-activate transcriptional activity of SRCs. MCB-613 markedly increases SRCs’ interactions with other coactivators. Coactivation of MMP2 or MMP13 promoter-driven luciferase reporter with SRC-3 was greatly enhanced by MCB-613. MCB-613 increased SRC-3’s interaction with CBP and CARM1 robustly in a dose-dependent manner. [1]
MCB-613 is cytotoxic. It can efficiently kill a variety of human cancer cell lines, including PC-3 (prostate), MCF-7 (breast), HepG2 (liver), and H1299 (lung)cells. Cancer cells overexpress SRCs and rely on them for cell growth, MCB-613 can selectively induce excessive ER stress coupled to the generation of reactive oxygen species (ROS) in cancer cells. [1]
MCB-613 treatment probably leads to a distinct phosphorylation pattern on SRC-3, which will be interesting to pursue in future studies. [1]
Reference:
Wang L, Yu Y, Chow DC et al. Characterization of a Steroid Receptor Coactivator Small Molecule Stimulator that Overstimulates Cancer Cells and Leads to Cell Stress and Death. Cancer Cell. 2015 Aug 10;28(2):240-52.
Lonard, D. M., and O’malley, B.W. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. 2007. Mol. Cell 27, 691–700.
Anzick, S. L., Kononen, J., Walker, R.L.et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. 1997, Science 277, 965–968.
- Levobetaxolol HCl
Catalog No.:BCC4671
CAS No.:116209-55-3
- Aflatoxin B1
Catalog No.:BCC9212
CAS No.:1162-65-8
- Complanatoside
Catalog No.:BCN8213
CAS No.:116183-66-5
- Alexine
Catalog No.:BCN2054
CAS No.:116174-63-1
- Brevicolline
Catalog No.:BCN2459
CAS No.:20069-02-7
- VU 0361737
Catalog No.:BCC4596
CAS No.:1161205-04-4
- G-15
Catalog No.:BCC6058
CAS No.:1161002-05-6
- Phenamil
Catalog No.:BCC7673
CAS No.:1161-94-0
- Z-Phe-OH
Catalog No.:BCC2756
CAS No.:1161-13-3
- RETF-4NA
Catalog No.:BCC6073
CAS No.:1160928-63-1
- IDE 1
Catalog No.:BCC7841
CAS No.:1160927-48-9
- Dehydromiltirone
Catalog No.:BCN5357
CAS No.:116064-77-8
- Pyrroside B
Catalog No.:BCN4042
CAS No.:116271-35-3
- 6-Aldehydoisoophiopogonanone A
Catalog No.:BCN2860
CAS No.:116291-82-8
- Clemizole hydrochloride
Catalog No.:BCC1486
CAS No.:1163-36-6
- Sarafotoxin S6b
Catalog No.:BCC5720
CAS No.:116303-65-2
- SKF 86002 dihydrochloride
Catalog No.:BCC7236
CAS No.:116339-68-5
- Fumonisin B1
Catalog No.:BCC2461
CAS No.:116355-83-0
- Acetylexidonin
Catalog No.:BCN3279
CAS No.:116368-90-2
- 2alpha-hydroxy-3beta-acetyloxy-betulic acid
Catalog No.:BCN3072
CAS No.:1163728-89-9
- Loureirin C
Catalog No.:BCN3761
CAS No.:116384-24-8
- 3',4',7-Trimethoxyflavan
Catalog No.:BCN6042
CAS No.:116384-26-0
- Z-Tyr-OH
Catalog No.:BCC2747
CAS No.:1164-16-5
- Androstanolone acetate
Catalog No.:BCC8826
CAS No.:1164-91-6
Molecular Pathways: Targeting Steroid Receptor Coactivators in Cancer.[Pubmed:27654711]
Clin Cancer Res. 2016 Nov 15;22(22):5403-5407.
Coactivators represent a large class of proteins that partner with nuclear receptors and other transcription factors to regulate gene expression. Given their pleiotropic roles in the control of transcription, coactivators have been implicated in a broad range of human disease states, including cancer. This is best typified by the three members of the steroid receptor coactivator (SRC) family, each of which integrates steroid hormone signaling and growth factor pathways to drive oncogenic gene expression programs in breast, endometrial, ovarian, prostate, and other cancers. Because of this, coactivators represent emerging targets for cancer therapeutics, and efforts are now being made to develop SRC-targeting agents, such as the SI-2 inhibitor and the novel SRC stimulator, MCB-613, that are able to block cancer growth in cell culture and animal model systems. Here, we will discuss the mechanisms through which coactivators drive cancer progression and how targeting coactivators represent a novel conceptual approach to combat tumor growth that is distinct from the use of other targeted therapeutic agents. We also will describe efforts to develop next-generation SRC inhibitors and stimulators that can be taken into the clinic for the treatment of recurrent, drug-resistant cancers. Clin Cancer Res; 22(22); 5403-7. (c)2016 AACR.
Characterization of a Steroid Receptor Coactivator Small Molecule Stimulator that Overstimulates Cancer Cells and Leads to Cell Stress and Death.[Pubmed:26267537]
Cancer Cell. 2015 Aug 10;28(2):240-52.
By integrating growth pathways on which cancer cells rely, steroid receptor coactivators (SRC-1, SRC-2, and SRC-3) represent emerging targets in cancer therapeutics. High-throughput screening for SRC small molecule inhibitors (SMIs) uncovered MCB-613 as a potent SRC small molecule "stimulator" (SMS). We demonstrate that MCB-613 can super-stimulate SRCs' transcriptional activity. Further investigation revealed that MCB-613 increases SRCs' interactions with other coactivators and markedly induces ER stress coupled to the generation of reactive oxygen species (ROS). Because cancer cells overexpress SRCs and rely on them for growth, we show that we can exploit MCB-613 to selectively induce excessive stress in cancer cells. This suggests that over-stimulating the SRC oncogenic program can be an effective strategy to kill cancer cells.


