RETF-4NASelective chymase substrate CAS# 1160928-63-1 |
- Radicicol
Catalog No.:BCC2131
CAS No.:12772-57-5
- NVP-BEP800
Catalog No.:BCC2129
CAS No.:847559-80-2
- BIIB021
Catalog No.:BCC2124
CAS No.:848695-25-0
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
- MPC-3100
Catalog No.:BCC2128
CAS No.:958025-66-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1160928-63-1 | SDF | Download SDF |
| PubChem ID | 90488886 | Appearance | Powder |
| Formula | C32H43N9O10 | M.Wt | 713.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in 20% acetonitrile / water | ||
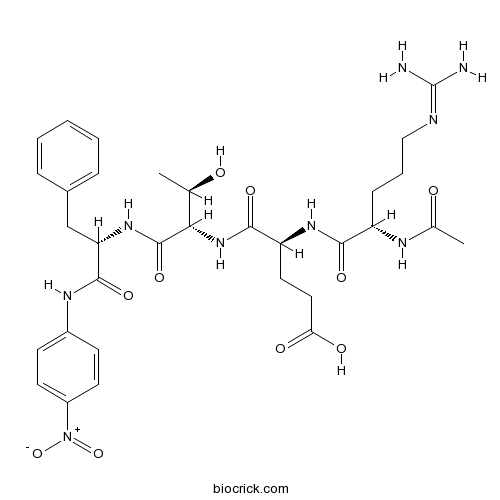
| Sequence | RETF (Modifications: Arg-1 = N-terminal Ac, Phe-4 = 4-Nitroanilide) | ||
| Chemical Name | (4S)-4-[[(2S)-2-acetamido-5-(diaminomethylideneamino)pentanoyl]amino]-5-[[(2S,3R)-3-hydroxy-1-[[(2S)-1-(4-nitroanilino)-1-oxo-3-phenylpropan-2-yl]amino]-1-oxobutan-2-yl]amino]-5-oxopentanoic acid | ||
| SMILES | CC(C(C(=O)NC(CC1=CC=CC=C1)C(=O)NC2=CC=C(C=C2)[N+](=O)[O-])NC(=O)C(CCC(=O)O)NC(=O)C(CCCN=C(N)N)NC(=O)C)O | ||
| Standard InChIKey | MGCWUIJMQAZRNY-SJYYQWPBSA-N | ||
| Standard InChI | InChI=1S/C32H43N9O10/c1-18(42)27(40-29(47)24(14-15-26(44)45)38-28(46)23(36-19(2)43)9-6-16-35-32(33)34)31(49)39-25(17-20-7-4-3-5-8-20)30(48)37-21-10-12-22(13-11-21)41(50)51/h3-5,7-8,10-13,18,23-25,27,42H,6,9,14-17H2,1-2H3,(H,36,43)(H,37,48)(H,38,46)(H,39,49)(H,40,47)(H,44,45)(H4,33,34,35)/t18-,23+,24+,25+,27+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Chymase substrate peptide that is cleaved more avidly by α2-macroglobulin-bound chymase than the free, unbound form. Displays selectivity over cathepsin G and chymotrypsin. |

RETF-4NA Dilution Calculator

RETF-4NA Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- IDE 1
Catalog No.:BCC7841
CAS No.:1160927-48-9
- Dehydromiltirone
Catalog No.:BCN5357
CAS No.:116064-77-8
- Piperolactam C
Catalog No.:BCN4818
CAS No.:116064-76-7
- Pyr3
Catalog No.:BCC7771
CAS No.:1160514-60-2
- MLN4924 HCl salt
Catalog No.:BCC1773
CAS No.:1160295-21-5
- VU 0238429
Catalog No.:BCC7729
CAS No.:1160247-92-6
- 1-Amino-4-hydroxyanthraquinone
Catalog No.:BCC8452
CAS No.:116-85-8
- 4-Amino-3-hydroxy-1-naphthalenesulfonic acid
Catalog No.:BCC8680
CAS No.:116-63-2
- Aldicarb
Catalog No.:BCC5475
CAS No.:116-06-3
- TC-I 2000
Catalog No.:BCC6244
CAS No.:1159996-20-9
- Caulophine
Catalog No.:BCN7990
CAS No.:1159989-19-1
- Abiesadine N
Catalog No.:BCN6041
CAS No.:1159913-80-0
- Z-Phe-OH
Catalog No.:BCC2756
CAS No.:1161-13-3
- Phenamil
Catalog No.:BCC7673
CAS No.:1161-94-0
- G-15
Catalog No.:BCC6058
CAS No.:1161002-05-6
- VU 0361737
Catalog No.:BCC4596
CAS No.:1161205-04-4
- Brevicolline
Catalog No.:BCN2459
CAS No.:20069-02-7
- Alexine
Catalog No.:BCN2054
CAS No.:116174-63-1
- Complanatoside
Catalog No.:BCN8213
CAS No.:116183-66-5
- Aflatoxin B1
Catalog No.:BCC9212
CAS No.:1162-65-8
- Levobetaxolol HCl
Catalog No.:BCC4671
CAS No.:116209-55-3
- MCB-613
Catalog No.:BCC3982
CAS No.:1162656-22-5
- Pyrroside B
Catalog No.:BCN4042
CAS No.:116271-35-3
- 6-Aldehydoisoophiopogonanone A
Catalog No.:BCN2860
CAS No.:116291-82-8
Alpha 2-macroglobulin capture allows detection of mast cell chymase in serum and creates a reservoir of angiotensin II-generating activity.[Pubmed:19380825]
J Immunol. 2009 May 1;182(9):5770-7.
Human chymase is a highly efficient angiotensin II-generating serine peptidase expressed by mast cells. When secreted from degranulating cells, it can interact with a variety of circulating antipeptidases, but is mostly captured by alpha(2)-macroglobulin, which sequesters peptidases in a cage-like structure that precludes interactions with large protein substrates and inhibitors, like serpins. The present work shows that alpha(2)-macroglobulin-bound chymase remains accessible to small substrates, including angiotensin I, with activity in serum that is stable with prolonged incubation. We used alpha(2)-macroglobulin capture to develop a sensitive, microtiter plate-based assay for serum chymase, assisted by a novel substrate synthesized based on results of combinatorial screening of peptide substrates. The substrate has low background hydrolysis in serum and is chymase-selective, with minimal cleavage by the chymotryptic peptidases cathepsin G and chymotrypsin. The assay detects activity in chymase-spiked serum with a threshold of approximately 1 pM (30 pg/ml), and reveals native chymase activity in serum of most subjects with systemic mastocytosis. alpha(2)-Macroglobulin-bound chymase generates angiotensin II in chymase-spiked serum, and it appears in native serum as chymostatin-inhibited activity, which can exceed activity of captopril-sensitive angiotensin-converting enzyme. These findings suggest that chymase bound to alpha(2)-macroglobulin is active, that the complex is an angiotensin-converting enzyme inhibitor-resistant reservoir of angiotensin II-generating activity, and that alpha(2)-macroglobulin capture may be exploited in assessing systemic release of secreted peptidases.


