Pyr3Selective TRPC3 blocker CAS# 1160514-60-2 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- CDK inhibitor II
Catalog No.:BCC1464
CAS No.:1269815-17-9
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1160514-60-2 | SDF | Download SDF |
| PubChem ID | 56964346 | Appearance | Powder |
| Formula | C16H11Cl3F3N3O3 | M.Wt | 456.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 125 mg/mL (273.74 mM) *"≥" means soluble, but saturation unknown. | ||
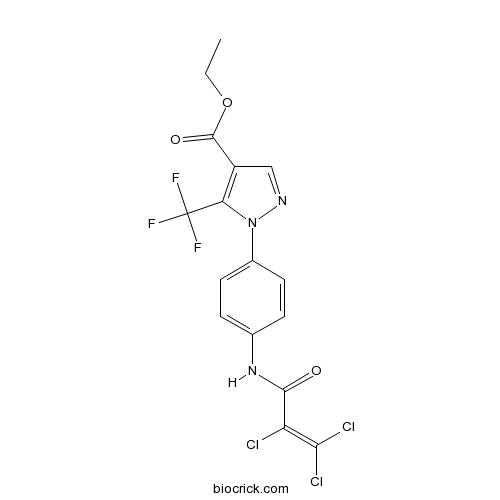
| Chemical Name | ethyl 1-[4-(2,3,3-trichloroprop-2-enoylamino)phenyl]-5-(trifluoromethyl)pyrazole-4-carboxylate | ||
| SMILES | CCOC(=O)C1=C(N(N=C1)C2=CC=C(C=C2)NC(=O)C(=C(Cl)Cl)Cl)C(F)(F)F | ||
| Standard InChIKey | RZHGONNSASQOAY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H11Cl3F3N3O3/c1-2-28-15(27)10-7-23-25(12(10)16(20,21)22)9-5-3-8(4-6-9)24-14(26)11(17)13(18)19/h3-7H,2H2,1H3,(H,24,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective antagonist of the canonical transient receptor potential channel 3 (TRPC3). Inhibits TRPC3-mediated Ca2+ influx (IC50 = 0.7 μM) and suppresses activation of nuclear factor of activated T cells (NFAT). Inhibits hypertrophic responses in cardiomyocytes. |

Pyr3 Dilution Calculator

Pyr3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.19 mL | 10.9498 mL | 21.8996 mL | 43.7991 mL | 54.7489 mL |
| 5 mM | 0.438 mL | 2.19 mL | 4.3799 mL | 8.7598 mL | 10.9498 mL |
| 10 mM | 0.219 mL | 1.095 mL | 2.19 mL | 4.3799 mL | 5.4749 mL |
| 50 mM | 0.0438 mL | 0.219 mL | 0.438 mL | 0.876 mL | 1.095 mL |
| 100 mM | 0.0219 mL | 0.1095 mL | 0.219 mL | 0.438 mL | 0.5475 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MLN4924 HCl salt
Catalog No.:BCC1773
CAS No.:1160295-21-5
- VU 0238429
Catalog No.:BCC7729
CAS No.:1160247-92-6
- 1-Amino-4-hydroxyanthraquinone
Catalog No.:BCC8452
CAS No.:116-85-8
- 4-Amino-3-hydroxy-1-naphthalenesulfonic acid
Catalog No.:BCC8680
CAS No.:116-63-2
- Aldicarb
Catalog No.:BCC5475
CAS No.:116-06-3
- TC-I 2000
Catalog No.:BCC6244
CAS No.:1159996-20-9
- Caulophine
Catalog No.:BCN7990
CAS No.:1159989-19-1
- Abiesadine N
Catalog No.:BCN6041
CAS No.:1159913-80-0
- CZC24832
Catalog No.:BCC1507
CAS No.:1159824-67-5
- Poricoic acid AE
Catalog No.:BCN7282
CAS No.:1159753-88-4
- Alstonic acid B
Catalog No.:BCN6040
CAS No.:1159579-45-9
- Alstonic acid A
Catalog No.:BCN6039
CAS No.:1159579-44-8
- Piperolactam C
Catalog No.:BCN4818
CAS No.:116064-76-7
- Dehydromiltirone
Catalog No.:BCN5357
CAS No.:116064-77-8
- IDE 1
Catalog No.:BCC7841
CAS No.:1160927-48-9
- RETF-4NA
Catalog No.:BCC6073
CAS No.:1160928-63-1
- Z-Phe-OH
Catalog No.:BCC2756
CAS No.:1161-13-3
- Phenamil
Catalog No.:BCC7673
CAS No.:1161-94-0
- G-15
Catalog No.:BCC6058
CAS No.:1161002-05-6
- VU 0361737
Catalog No.:BCC4596
CAS No.:1161205-04-4
- Brevicolline
Catalog No.:BCN2459
CAS No.:20069-02-7
- Alexine
Catalog No.:BCN2054
CAS No.:116174-63-1
- Complanatoside
Catalog No.:BCN8213
CAS No.:116183-66-5
- Aflatoxin B1
Catalog No.:BCC9212
CAS No.:1162-65-8
Pyr3, a TRPC3 channel blocker, potentiates dexamethasone sensitivity and apoptosis in acute lymphoblastic leukemia cells by disturbing Ca(2+) signaling, mitochondrial membrane potential changes and reactive oxygen species production.[Pubmed:27179991]
Eur J Pharmacol. 2016 Aug 5;784:90-8.
Dexamethasone (Dex) is used as a chemotherapeutic drug in the treatment of acute lymphoblastic leukemia (ALL) because of its capacity to induce apoptosis. However, some ALL patients acquire resistance to glucocorticoids (GC). Thus, it is important to explore new agents to overcome GC resistance. The aim of the present work was to assess the ability of Pyr3, a selective inhibitor of transient receptor potential canonical 3 (TRPC3), to sensitize human ALL cells to Dex. We show here, for the first time, that Pyr3 enhances Dex sensitivity through the distraction of Dex-mediated Ca(2+) signaling in ALL cells (in vitro) and primary blasts (ex vivo) associated with mitochondrial-mediated reactive oxygen species production in ALL cells. Pyr3 alone induced Ca(2+) signaling via only endoplasmic reticulum-released Ca(2+) and exerted inhibitory effect on store-operated Ca(2+) entry in dose-dependent manner in ALL cell lines. Pre-incubation of cells with Pyr3 significantly curtailed the thapsigargin- and Dex-evoked Ca(2+) signaling in ALL cell lines. Pyr3 synergistically potentiated Dex lethality, as shown by the induction of cell mortality, G2/M cell cycle arrest and apoptosis in ALL cell lines. Moreover, Pyr3 disrupted Dex-mediated Ca(2+) signaling and increased the sensitivity of Dex-induced cell death in primary blasts from ALL patients. Additional analysis showed that co-treatment with Dex and Pyr3 results in mitochondrial membrane potential depolarization and reactive oxygen species production in ALL cells. Together, Pyr3 exhibited potential therapeutic benefit in combination with Dex to inverse glucocorticoid resistance in human ALL and probably in other lymphoid malignancies.
A TRPC3 blocker, ethyl-1-(4-(2,3,3-trichloroacrylamide)phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-c arboxylate (Pyr3), prevents stent-induced arterial remodeling.[Pubmed:23010361]
J Pharmacol Exp Ther. 2013 Jan;344(1):33-40.
TRPC-mediated Ca(2+) entry has been implicated in the control of smooth muscle proliferation and might represent a pivotal mechanism underlying in-stent restenosis. As we have observed significant expression of TRPC3 in human smooth muscle from the coronary artery as well as the aorta, we tested the efficiency of a recently discovered TRPC3 selective Ca(2+) entry blocker Pyr3 to prevent vascular smooth muscle proliferation and stent implantation-induced hyperplasia of human aorta. The effect of Pyr3 on proliferation was measured by detection of BrdU incorporation and PCNA expression in human coronary smooth muscle and microvascular endothelium, which displays significantly smaller expression levels of TRPC3 as compared with smooth muscle. Pyr3 inhibited smooth muscle proliferation but lacked detectable effects on endothelial proliferation. Measurements of ATP-induced Ca(2+) signals revealed that Pyr3 suppressed agonist-induced Ca(2+) entry more effectively in vascular smooth muscle than in endothelial cells. Inhibitory effects of Pyr3 on stent implantation-induced arterial injury was tested using a novel in vitro model of in-stent hyperplasia in human arteries based on organ typical culture of human aortic constructs. Pyr3 effectively prevented increases in tissue levels of PCNA and Ki-67 at 2 weeks after stent implantation into human aortae. Similarly, proliferation markers were significantly suppressed when implanting a Pyr3-releasing stent prototype as compared with a bare metal stent (BMS) control. Our results suggest TRPC3 as a potential target for pharmacological control of smooth muscle proliferation. Selectively inhibition of TRPC Ca(2+) entry channels in vascular smooth muscle is suggested as a promising strategy for in-stent restenosis prevention.
Transient receptor potential canonical 3 inhibitor Pyr3 improves outcomes and attenuates astrogliosis after intracerebral hemorrhage in mice.[Pubmed:23674527]
Stroke. 2013 Jul;44(7):1981-7.
BACKGROUND AND PURPOSE: Intracerebral hemorrhage (ICH) stems from the rupture of blood vessels in the brain, with the subsequent accumulation of blood in the parenchyma. Increasing evidence suggests that blood-derived factors induce excessive inflammatory responses that are involved in the progression of ICH-induced brain injury. Thrombin, a major blood-derived factor, leaks into the brain parenchyma on blood-brain barrier disruption and induces brain injury and astrogliosis. Furthermore, thrombin dynamically upregulates transient receptor potential canonical 3 channel, which contributes to pathological astrogliosis through a feed-forward upregulation of its own expression. The present study investigated whether Ethyl-1-(4-(2,3,3-trichloroacrylamide)phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-c arboxylate (Pyr3), a specific transient receptor potential canonical 3 inhibitor, can improve functional outcomes and attenuate astrogliosis after ICH in mice. METHODS: Male C57BL6 mice received an intracerebral infusion of collagenase or autologous blood to induce ICH. Pyr3 was given both intracerebroventricularly and intraperitoneally after ICH induction. ICH-induced brain injury was evaluated by quantitative assessment of neurological deficits, brain swelling, and injury volume after ICH. Astrocyte activation was evaluated by immunohistochemical assessment of changes in S100 protein expression. RESULTS: Neurological deficits, neuronal injury, brain edema, and astrocyte activation were all significantly improved by administration of Pyr3. Moreover, delayed administration of Pyr3 at 6 hours or 1 day after blood or collagenase infusion, respectively, also improved the symptoms. CONCLUSIONS: Pyr3, a specific inhibitor of transient receptor potential canonical 3, reduced the perihematomal accumulation of astrocytes and ameliorated ICH-induced brain injury. Therefore, transient receptor potential canonical 3 provides a new therapeutic target for the treatment of hemorrhagic brain injury.


