WAY 213613Potent, non-substrate EAAT2 inhibitor CAS# 868359-05-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 868359-05-1 | SDF | Download SDF |
| PubChem ID | 11531745 | Appearance | Powder |
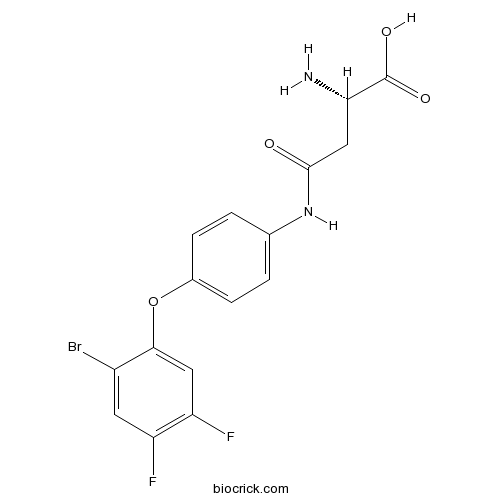
| Formula | C16H13BrF2N2O4 | M.Wt | 415.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH and to 100 mM in DMSO | ||
| Chemical Name | (2S)-2-amino-4-[4-(2-bromo-4,5-difluorophenoxy)anilino]-4-oxobutanoic acid | ||
| SMILES | C1=CC(=CC=C1NC(=O)CC(C(=O)O)N)OC2=CC(=C(C=C2Br)F)F | ||
| Standard InChIKey | BNYDDAAZMBUFRG-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C16H13BrF2N2O4/c17-10-5-11(18)12(19)6-14(10)25-9-3-1-8(2-4-9)21-15(22)7-13(20)16(23)24/h1-6,13H,7,20H2,(H,21,22)(H,23,24)/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, non-substrate inhibitor of EAAT2 (GLT-1) that displays > 44-fold selectivity over EAAT1 and EAAT3 (IC50 values are 85, 3787 and 5004 nM for EAAT2, EAAT3 and EAAT1 respectively). Exhibits no activity towards ionotropic and metabotropic glutamate receptors. |

WAY 213613 Dilution Calculator

WAY 213613 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4085 mL | 12.0427 mL | 24.0854 mL | 48.1707 mL | 60.2134 mL |
| 5 mM | 0.4817 mL | 2.4085 mL | 4.8171 mL | 9.6341 mL | 12.0427 mL |
| 10 mM | 0.2409 mL | 1.2043 mL | 2.4085 mL | 4.8171 mL | 6.0213 mL |
| 50 mM | 0.0482 mL | 0.2409 mL | 0.4817 mL | 0.9634 mL | 1.2043 mL |
| 100 mM | 0.0241 mL | 0.1204 mL | 0.2409 mL | 0.4817 mL | 0.6021 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rhodiosin; Herbacetin-7-O-glucorhamnoside
Catalog No.:BCN8478
CAS No.:86831-54-1
- Rhodiolin
Catalog No.:BCC8356
CAS No.:86831-53-0
- Org 27569
Catalog No.:BCC4411
CAS No.:868273-06-7
- LY 2365109 hydrochloride
Catalog No.:BCC7677
CAS No.:868265-28-5
- Pam2CSK4
Catalog No.:BCC6247
CAS No.:868247-72-7
- SID 7969543
Catalog No.:BCC6026
CAS No.:868224-64-0
- Carasiphenol C
Catalog No.:BCN8251
CAS No.:868168-04-1
- H-Cys-OEt.HCl
Catalog No.:BCC2904
CAS No.:868-59-7
- CRF (human, rat)
Catalog No.:BCC5710
CAS No.:86784-80-7
- Astrasieversianin VII
Catalog No.:BCN2788
CAS No.:86764-11-6
- TG 100572
Catalog No.:BCC1994
CAS No.:867334-05-2
- TG 100801
Catalog No.:BCC1996
CAS No.:867331-82-6
- 2,2,5,5-Tetramethylcyclohexane-1,4-dione
Catalog No.:BCN1324
CAS No.:86838-54-2
- Protosappanin A dimethyl acetal
Catalog No.:BCN6517
CAS No.:868405-37-2
- (+)-Puerol B 2''-O-glucoside
Catalog No.:BCN4561
CAS No.:868409-19-2
- Iristectorigenin B
Catalog No.:BCN8391
CAS No.:39012-01-6
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
- 3',5'-Dimethoxybiphenyl-3-ol
Catalog No.:BCN7529
CAS No.:868666-20-0
- (R)-(-)-α-Methylhistamine dihydrobromide
Catalog No.:BCC5665
CAS No.:868698-49-1
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
- Eudesma-3,11-dien-2-one
Catalog No.:BCN7607
CAS No.:86917-79-5
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
- Neurokinin A (porcine)
Catalog No.:BCC6955
CAS No.:86933-74-6
Achalasia-an unnecessary long way to diagnosis.[Pubmed:28375437]
Dis Esophagus. 2017 May 1;30(5):1-6.
Although achalasia presents with typical symptoms such as dysphagia, regurgitation, weight loss, and atypical chest pain, the time until first diagnosis often takes years and is frustrating for patients and nevertheless associated with high costs for the healthcare system. A total of 563 patients were interviewed with confirmed diagnosis of achalasia regarding their symptoms leading to diagnosis along with past clinical examinations and treatments. Included were patients who had undergone their medical investigations in Germany. Overall, 527 study subjects were included (male 46%, female 54%, mean age at time of interview 51 +/- 14.8 years). Dysphagia was present in 86.7%, regurgitation in 82.9%, atypical chest pain in 79%, and weight loss in 58% of patients before diagnosis. On average, it took 25 months (Interquartile Range (IQR) 9-65) until confirmation of correct diagnosis of achalasia. Though, diagnosis was confirmed significantly quicker (35 months IQR 9-89 vs. 20 months IQR 8-53; p < 0.01) in the past 15 years. The majority (72.1%) was transferred to three or more specialists. Almost each patient underwent at least one esophagogastroduodenoscopy (94.2%) and one radiological assessment (89.3%). However, esophageal manometry was performed in 70.4% of patients only. The severity of symptoms was independent with regard to duration until first diagnosis (Eckardt score 7.14 +/- 2.64 within 12 months vs. 7.29 +/- 2.61 longer than 12 months; P = 0.544). Fifty-five percent of the patients primarily underwent endoscopic dilatation and 37% a surgical myotomy. Endoscopic dilatation was realized significantly faster compared to esophageal myotomy (1 month IQR 0-4 vs. 3 months IQR 1-11; p < 0.001). Although diagnosis of achalasia was significantly faster in the past 15 years, it still takes almost 2 years until the correct diagnosis of achalasia is confirmed. Alarming is the fact that although esophageal manometry is known as the gold standard to differentiate primary motility disorders, only three out of four patients had undergone this diagnostic pathway during their diagnostic work-up. Better education of medical professionals and broader utilization of highly sensitive diagnostic tools, such as high-resolution manometry, are strictly necessary in order to correctly diagnose affected patients and to offer therapy faster.
C-Tactile Mediated Erotic Touch Perception Relates to Sexual Desire and Performance in a Gender-Specific Way.[Pubmed:28372939]
J Sex Med. 2017 May;14(5):645-653.
BACKGROUND: Unmyelinated low-threshold mechanoreceptors-the so-called C-tactile (CT) afferents-play a crucial role in the perception and conduction of caressing and pleasant touch sensations and significantly contribute to the concept of erotic touch perception. AIM: To investigate the relations between sexual desire and sexual performance and the perception of touch mediated by CT afferents. METHODS: Seventy healthy participants (28 men, 42 women; mean age +/- SD = 24.84 +/- 4.08 years, range = 18-36 years) underwent standardized and highly controlled stroking stimulation that varied in the amount of CT fiber stimulation by changing stroking velocity (CT optimal = 1, 3 and 10 cm/s; CT suboptimal = 0.1, 0.3, and 30 cm/s). Participants rated the perceived pleasantness, eroticism, and intensity of the applied tactile stimulation on a visual analog scale, completed the Sexual Desire Inventory, and answered questions about sexual performance. OUTCOMES: Ratings of perceived eroticism of touch were related to self-report levels of sexual desire and sexual performance. RESULTS: Pleasantness and eroticism ratings showed similar dependence on stroking velocity that aligned with the activity of CT afferents. Erotic touch perception was related to sexual desire and sexual performance in a gender-specific way. In women, differences in eroticism ratings between CT optimal and suboptimal velocities correlated positively with desire for sexual interaction. In contrast, in men, this difference correlated to a decreased frequency and longer duration of partnered sexual activities. CLINICAL IMPLICATIONS: The present results lay the foundation for future research assessing these relations in patients with specific impairments of sexual functioning (eg, hypoactive sexual desire disorder). STRENGTHS AND LIMITATIONS: The strength of the study is the combination of standardized neurophysiologic methods and behavioral data. A clear limitation of the study design is the exclusion of exact data on the female menstrual cycle and the recruitment of an inhomogeneous sample concerning sexual orientation. CONCLUSION: The present results provide further evidence that unmyelinated CT afferents play a role in the complex mechanism of erotic touch perception. The ability to differentiate between CT optimal and suboptimal stimuli relates to sexual desire and performance in a gender-specific way. Bendas J, Georgiadis JR, Ritschel G. C-Tactile Mediated Erotic Touch Perception Relates to Sexual Desire and Performance in a Gender-Specific Way. J Sex Med 2017;14:645-653.
A sensitive ratiometric electrochemical biosensor based on DNA four-way junction formation and enzyme-assisted recycling amplification.[Pubmed:28379274]
Analyst. 2017 May 2;142(9):1562-1568.
A simple ratiometric electrochemical biosensor is developed for sensitive detection of target DNA based on DNA four-way junction (DNA-4WJ) formation and enzyme-assisted recycling amplification. This biosensor can be easily fabricated by a one-step assembly of ratiometric probes and simply performed by a one-step incubation procedure. In the presence of target DNA, two unmodified DNA oligonucleotides may cooperatively hybridize with a hairpin probe in the triple-helix molecular beacon (THMB) to form a DNA-4WJ, which may cause conformational transduction and induce the change in the distance between two redox labeling probes and the electrode surface. The subsequent recognition and cleavage of DNA-4WJ quadripartite complexes by RNase HII may result in significant signal amplification. Due to the introduction of DNA-4WJ formation, enzyme-assisted recycling amplification and ratiometric measurement, this biosensor exhibits high sensitivity with a detection limit as low as 0.063 pM and a long dynamic range from 0.1 pM to 100 nM. Moreover, this biosensor demonstrates good performance with excellent selectivity, high reliability and good reproducibility, holding great potential for further applications in biomedical research and clinical diagnostics.
The problem of choice: From the voluntary way to Affordable Care Act health insurance exchanges.[Pubmed:28371627]
Soc Sci Med. 2017 May;181:34-42.
This article takes a genealogical and ethnographic approach to the problem of choice, arguing that what choice means has been reworked several times since health insurance first figured prominently in national debates about health reform. Whereas voluntary choice of doctor and hospital used to be framed as an American right, contemporary choice rhetoric includes consumer choice of insurance plan. Understanding who has deployed choice rhetoric and to what ends helps explain how offering choices has become the common sense justification for defending and preserving the exclusionary health care system in the United States. Four case studies derived from 180 enrollment observations at the Rhode Island health insurance exchange conducted from March 2014-January 2017 and interviews with enrollees show how choice is experienced in this latest iteration of health reform. The Affordable Care Act (ACA) of 2010 created new pathways to insurance coverage in the United States. Insurance exchanges were supposed to unleash the power of consumer decision-making through marketplaces where health plans compete on quality, coverage, and price. Consumers, however, contended with confusing insurance terminology and difficult to navigate websites. The ethnography shows that consumers experienced choice as confusing and overwhelming and did not feel "in charge" of their decisions. Instead, unstable employment, changes in income, existing health needs, and bureaucratic barriers shaped their "choices."
Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement.[Pubmed:17088867]
Br J Pharmacol. 2007 Jan;150(1):5-17.
L-Glutamate (Glu) is the major excitatory neurotransmitter in the mammalian CNS and five types of high-affinity Glu transporters (EAAT1-5) have been identified. The transporters EAAT1 and EAAT2 in glial cells are responsible for the majority of Glu uptake while neuronal EAATs appear to have specialized roles at particular types of synapses. Dysfunction of EAATs is specifically implicated in the pathology of neurodegenerative conditions such as amyotrophic lateral sclerosis, epilepsy, Huntington's disease, Alzheimer's disease and ischemic stroke injury, and thus treatments that can modulate EAAT function may prove beneficial in these conditions. Recent advances have been made in our understanding of the regulation of EAATs, including their trafficking, splicing and post-translational modification. This article summarises some recent developments that improve our understanding of the roles and regulation of EAATs.
Characterization of novel aryl-ether, biaryl, and fluorene aspartic acid and diaminopropionic acid analogs as potent inhibitors of the high-affinity glutamate transporter EAAT2.[Pubmed:16014807]
Mol Pharmacol. 2005 Oct;68(4):974-82.
In this study, we describe the pharmacological characterization of novel aryl-ether, biaryl, and fluorene aspartic acid and diaminopropionic acid analogs as potent inhibitors of EAAT2, the predominant glutamate transporter in forebrain regions. The rank order of potency determined for the inhibition of human EAAT2 was N(4)-[4-(2-bromo-4,5-difluorophenoxy)phenyl]-L-asparagine (WAY-213613) (IC(50) = 85 +/- 5 nM) > N(4)-(2'-methyl-1,1'-biphenyl-4-yl)-L-asparagine (WAY-213394) (IC(50) = 145 +/- 22 nM) = N(4)-[7-(trifluoromethyl)-9H-fluoren-2-yl]-L-asparagine (WAY-212922) (IC(50) = 157 +/- 11 nM) = 3-{[(4'-chloro-2-methyl-1,1'-biphenyl-4-yl)carbonyl]amino}-L-alanine (WAY-211686) (IC(50) = 190 +/- 10 nM). WAY-213613 was the most selective of the compounds examined, with IC(50) values for inhibition of EAAT1 and EAAT3 of 5 and 3.8 microM, respectively, corresponding to a 59- and 45-fold selectivity toward EAAT2. An identical rank order of potency [WAY-213613 (35 +/- 7 nM) > WAY-213394 (92 +/- 13 nM) = WAY-212922 (95 +/- 8 nM) = WAY-211686 (101 +/- 20 nM)] was observed for the inhibition of glutamate uptake in rat cortical synaptosomes, consistent with the predominant contribution of EAAT2 to this activity. Kinetic studies with each of the compounds in synaptosomes revealed a competitive mechanism of inhibition. All compounds were determined to be nonsubstrates by evaluating both the stimulation of currents in EAAT2-injected oocytes and the heteroexchange of d-[(3)H]aspartate from cortical synaptosomes. WAY-213613 represents the most potent and selective inhibitor of EAAT2 identified to date. Taken in combination with its selectivity over ionotropic and metabotropic glutamate receptors, this compound represents a potential tool for the further elucidation of EAAT2 function.


