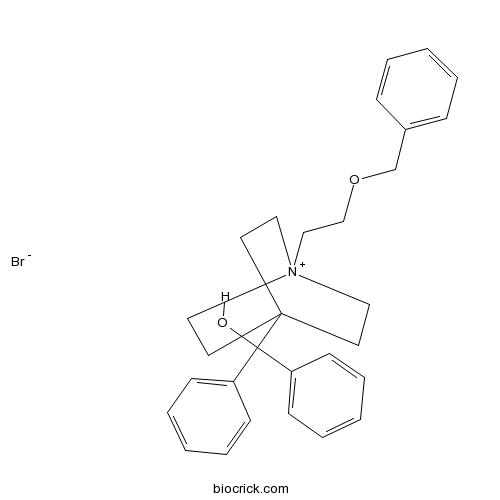
Umeclidinium bromideMAChR antagonist CAS# 869113-09-7 |
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
- SU5416
Catalog No.:BCC1974
CAS No.:204005-46-9
- L-Kynurenine
Catalog No.:BCC3899
CAS No.:2922-83-0
- CH 223191
Catalog No.:BCC3896
CAS No.:301326-22-7
- ITE
Catalog No.:BCC3902
CAS No.:448906-42-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 869113-09-7 | SDF | Download SDF |
| PubChem ID | 11519069 | Appearance | Powder |
| Formula | C29H34BrNO2 | M.Wt | 508.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GSK573719A | ||
| Solubility | DMSO : ≥ 34 mg/mL (66.86 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | diphenyl-[1-(2-phenylmethoxyethyl)-1-azoniabicyclo[2.2.2]octan-4-yl]methanol;bromide | ||
| SMILES | C1C[N+]2(CCC1(CC2)C(C3=CC=CC=C3)(C4=CC=CC=C4)O)CCOCC5=CC=CC=C5.[Br-] | ||
| Standard InChIKey | PEJHHXHHNGORMP-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C29H34NO2.BrH/c31-29(26-12-6-2-7-13-26,27-14-8-3-9-15-27)28-16-19-30(20-17-28,21-18-28)22-23-32-24-25-10-4-1-5-11-25;/h1-15,31H,16-24H2;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Umeclidinium bromide is a novel mAChR antagonist. The affinity (Ki) of Umeclidinium bromide for the cloned human M1-M5 mAChRs ranges from 0.05 to 0.16 nM.In Vitro:In human embryonic kidney 293 cells, Umeclidinium bromide (GSK573719A) inhibits the human ether-a-go-go-related gene channel tail current in a concentration-dependent manner (IC50=9.4 μM)[1]. Umeclidinium bromide, previously known as GSK573719, is a novel high-affinity specific mAChR antagonist. It is a potent agent that demonstrates slow functional reversibility at cloned human M3 mAChRs and at endogenous mAChR in isolated human bronchus[2].In Vivo:When Umeclidinium bromide (GSK573719A) is given once daily to mice for 5 consecutive days (0.025 μg intranasally), the level of inhibition on the fifth day is modestly increased above that obtained after a single administration to the same mice (60 versus 35%, respectively). After the fifth day of dosing, the mice are rested for 5 additional days, allowing bronchomotor tone to return to baseline levels. On the sixth day, the mice receive one last dose of antagonist and are once again challenged with Mch. The level of inhibition is essentially the same as that found on the first day of testing, indicating that tolerance is not evident with repeated intranasal delivery of Umeclidinium bromide. By contrast, when Umeclidinium bromide is given orally (2.0 mg/kg) to mice at a dose 100 times the ED50 value (intranasal), there is no observable protection against an Mch challenge[1]. References: | |||||

Umeclidinium bromide Dilution Calculator

Umeclidinium bromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9666 mL | 9.833 mL | 19.6661 mL | 39.3321 mL | 49.1652 mL |
| 5 mM | 0.3933 mL | 1.9666 mL | 3.9332 mL | 7.8664 mL | 9.833 mL |
| 10 mM | 0.1967 mL | 0.9833 mL | 1.9666 mL | 3.9332 mL | 4.9165 mL |
| 50 mM | 0.0393 mL | 0.1967 mL | 0.3933 mL | 0.7866 mL | 0.9833 mL |
| 100 mM | 0.0197 mL | 0.0983 mL | 0.1967 mL | 0.3933 mL | 0.4917 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Umeclidinium bromide is a potent and long-acting antagonist of muscarinic cholinergic receptor (mAChR) with Ki values of 0.16nM, 0.15nM, 0.06nM, 0.05nM and 0.13nM for M1, M2, M3, M4 and M5, respectively [1].
Umeclidinium bromide is developed for the treatment of chronic obstructive pulmonary disease (COPD). It can help patients of COPD achieve sufficient oxygenation of extrapulmonary tissues through dilating the airways. In the in vitro assay, umeclidinium binds to recombinant human mAChRs with Ki value of 0.16nM, 0.15nM, 0.06nM, 0.05nM and 0.13nM for M1-M5, respectively. Umeclidinium is selective against mAChR over other unrelated receptors or channels such as κ and σ opiod receptors, Na+ channel and dopamine transporter. In CHO cells transfected with human recombinant mAChRs, umeclidinium affects the calcium flux responsed to Ach with pA2 values of 9.6-10.6 for M1-M3. In a murine model, administration of umeclidinium can reverse the bronchoconstriction caused by Ach [1].
References:
[1] Salmon M, Luttmann M A, Foley J J, et al. Pharmacological characterization of GSK573719 (umeclidinium): a novel, long-acting, inhaled antagonist of the muscarinic cholinergic receptors for treatment of pulmonary diseases. Journal of Pharmacology and Experimental Therapeutics, 2013, 345(2): 260-270.
- (R)-(-)-α-Methylhistamine dihydrobromide
Catalog No.:BCC5665
CAS No.:868698-49-1
- 3',5'-Dimethoxybiphenyl-3-ol
Catalog No.:BCN7529
CAS No.:868666-20-0
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
- Iristectorigenin B
Catalog No.:BCN8391
CAS No.:39012-01-6
- (+)-Puerol B 2''-O-glucoside
Catalog No.:BCN4561
CAS No.:868409-19-2
- Protosappanin A dimethyl acetal
Catalog No.:BCN6517
CAS No.:868405-37-2
- 2,2,5,5-Tetramethylcyclohexane-1,4-dione
Catalog No.:BCN1324
CAS No.:86838-54-2
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- Rhodiosin; Herbacetin-7-O-glucorhamnoside
Catalog No.:BCN8478
CAS No.:86831-54-1
- Rhodiolin
Catalog No.:BCC8356
CAS No.:86831-53-0
- Org 27569
Catalog No.:BCC4411
CAS No.:868273-06-7
- LY 2365109 hydrochloride
Catalog No.:BCC7677
CAS No.:868265-28-5
- Eudesma-3,11-dien-2-one
Catalog No.:BCN7607
CAS No.:86917-79-5
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
- Neurokinin A (porcine)
Catalog No.:BCC6955
CAS No.:86933-74-6
- Neurokinin B (human, porcine)
Catalog No.:BCC7119
CAS No.:86933-75-7
- AZD8330
Catalog No.:BCC3733
CAS No.:869357-68-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- 14-Deoxy-17-hydroxyandrographolide
Catalog No.:BCN4560
CAS No.:869384-82-7
- Praeroside II
Catalog No.:BCN7001
CAS No.:86940-46-7
- JNJ 10191584 maleate
Catalog No.:BCC7362
CAS No.:869497-75-6
- Tecovirimat
Catalog No.:BCC5518
CAS No.:869572-92-9
- Fmoc-Tyr(tBu)-ol
Catalog No.:BCC2572
CAS No.:86967-51-3
Umeclidinium bromide/vilanterol combination in the treatment of chronic obstructive pulmonary disease: a review.[Pubmed:25848294]
Ther Clin Risk Manag. 2015 Mar 25;11:481-7.
Chronic obstructive pulmonary disease (COPD) is a common disease among the elderly that could be prevented by smoking cessation. As it is characterized by airflow limitation that is not fully reversible, bronchodilator therapy is the first choice of treatment. Symptomatic COPD patients with or without risk for future exacerbations have a strong indication for the permanent use of long- and ultralong-acting beta2-agonists and/or long-acting muscarinic antagonists. Combining bronchodilators is an effective approach, as they demonstrate synergic action at a cellular level and have additive clinical benefits and fewer adverse events compared with increased doses of the monocomponents. Novel fixed-dose combinations of long-acting beta2-agonists/long-acting muscarinic antagonists in one inhaler have been approved for clinical use by the US Food and Drug Administration and the European Medicines Agency. This review focuses on published clinical trials about the fixed-dose combination of umeclidinium/vilanterol trifenatate in patients with COPD. Results from six studies (five of them of 12 weeks' duration and one that lasted 1 year, including more than 6,000 patients in total) showed that umeclidinium/vilanterol trifenatate improved lung function, dyspnea, and health-related quality of life and decreased the exacerbation rate with no serious adverse events. More longstanding trials are needed to evaluate the effect of the drug on disease progression and compare it directly with other fixed-dose combinations.
Umeclidinium bromide + vilanterol for the treatment of chronic obstructive pulmonary disease.[Pubmed:25382021]
Expert Rev Clin Pharmacol. 2015 Jan;8(1):35-41.
A solid scientific rationale and an increasing body of clinical evidence fully support the use of an antimuscarinic agent combined with a beta-agonist in chronic obstructive pulmonary disease. In this article, we focus on the development of an inhaled fixed dose combination (FDC) of two 24-h bronchodilators, Umeclidinium bromide and vilanterol (UMEC/VI) (ANORO). Several pivotal clinical trials have documented the impact of this combination on lung function and other outcome measures such as quality of life, dyspnea, rescue medication use and exercise capacity, with no clinically meaningful treatment-related changes in vital signs or clinical laboratory parameters. These results allow us to predict that UMEC/VI will have a role in the maintenance treatment of chronic obstructive pulmonary disease. It remains to determine its impact on exacerbations. In any case, trials comparing UMEC/VI with other dual bronchodilator FDCs, and also with inhaled corticosteroid/long-acting beta-agonist FDCs, are needed to assess the advantages, if any, of UMEC/VI FDC over other therapies.


