PF-573228ATP-competitive FAK inhibitor CAS# 869288-64-2 |
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- CEP-37440
Catalog No.:BCC5145
CAS No.:1391712-60-9
- PF-562271
Catalog No.:BCC3674
CAS No.:717907-75-0
- TAE226 (NVP-TAE226)
Catalog No.:BCC3885
CAS No.:761437-28-9
- PF-00562271
Catalog No.:BCC3684
CAS No.:939791-38-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 869288-64-2 | SDF | Download SDF |
| PubChem ID | 11612883 | Appearance | Powder |
| Formula | C22H20F3N5O3S | M.Wt | 491.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 51 mg/mL (103.77 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
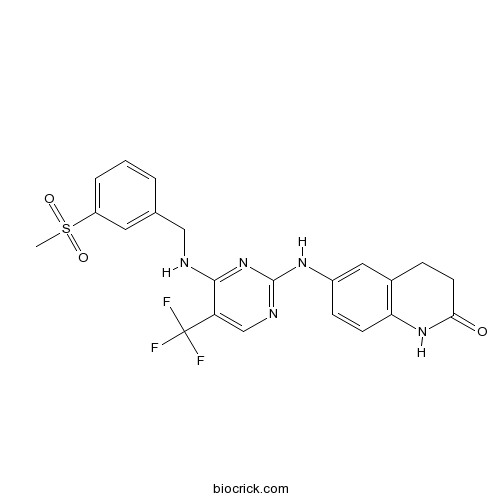
| Chemical Name | 6-[[4-[(3-methylsulfonylphenyl)methylamino]-5-(trifluoromethyl)pyrimidin-2-yl]amino]-3,4-dihydro-1H-quinolin-2-one | ||
| SMILES | CS(=O)(=O)C1=CC=CC(=C1)CNC2=NC(=NC=C2C(F)(F)F)NC3=CC4=C(C=C3)NC(=O)CC4 | ||
| Standard InChIKey | HESLKTSGTIBHJU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective inhibitor of focal adhesion kinase (FAK) (IC50 = 4 nM). Displays 50 - 250-fold selectivity for FAK over other protein kinases. Blocks serum and fibronectin-directed migration and decreases focal adhesion turnover in vitro. |

PF-573228 Dilution Calculator

PF-573228 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0346 mL | 10.1731 mL | 20.3463 mL | 40.6926 mL | 50.8657 mL |
| 5 mM | 0.4069 mL | 2.0346 mL | 4.0693 mL | 8.1385 mL | 10.1731 mL |
| 10 mM | 0.2035 mL | 1.0173 mL | 2.0346 mL | 4.0693 mL | 5.0866 mL |
| 50 mM | 0.0407 mL | 0.2035 mL | 0.4069 mL | 0.8139 mL | 1.0173 mL |
| 100 mM | 0.0203 mL | 0.1017 mL | 0.2035 mL | 0.4069 mL | 0.5087 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF573228 is an inhibitor of FAK with IC50 value of 4 nM [1].
FAK (Focal adhesion kinase) is a non-receptor protein-tyrosine kinase that resides at the sites of focal adhesions. FAK protein is encoded by the FAK gene located on human chromosome 8q24 and the molecular weight is 125 kDa. FAK works as an important mediator of cell activities, like cell adhesion, growth, proliferation, survival, angiogenesis and migration, it is reported that normal tissues FAK are significantly lower than in primary and metastatic tumors [2].
PF573228 is a specific inhibitor of FAK and is regarded as potent anti-angiogenic agents. When tested with HUVEC (primary human umbilical vein endothelial cells), PF573228 treatment increased the proportion of cell apoptosis, reduced the ability of endothelial cell migration and sprout formation via inhibiting the autophosphorylation of FAK [3]. In A431 epithelial carcinoma cells, incubation with PF573228 resulted in the reduced phosphorylation of FAK with IC50 value of 11 nM and PF573228 also observed to inhibit the FAK phosphorylation in many other cancer cells, such as PC3 cells, SKOV-3 cells, L3.6p1 cells, F-G cells and MDCK cells with IC50 value of 30-500 nM [1]. When tested with MSCs (mesenchymal stem cells), PF573228 treatment depressed the MSCs pro-inflammatory response to CM from FaDu, MDA-MB-231, PC-3 and NCI-H522 via inhibiting FAK phosphorylation [4].
PF573228 is also reported functioned in the process of BK (Ca)-channel. When tested with pituitary tumor (GH (3)) cells transfected with K (Ca) 1.1 siRNAs, 3 uM PF573228 treatment stimulated the BK (Ca)-channel activity which subsequently may influence cell behavior [5].
References:
[1]. Slack-Davis, J.K., et al., Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem, 2007. 282(20): p. 14845-52.
[2]. Golubovskaya, V.M., Focal adhesion kinase as a cancer therapy target. Anticancer Agents Med Chem, 2010. 10(10): p. 735-41.
[3]. Cabrita, M.A., et al., Focal adhesion kinase inhibitors are potent anti-angiogenic agents. Mol Oncol, 2011. 5(6): p. 517-26.
[4]. Al-toub, M., et al., Pleiotropic effects of cancer cells' secreted factors on human stromal (mesenchymal) stem cells. Stem Cell Res Ther, 2013. 4(5): p. 114.
[5]. So, E.C., et al., Evidence for activation of BK Ca channels by a known inhibitor of focal adhesion kinase, PF573228. Life Sci, 2011. 89(19-20): p. 691-701.
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
- Eudesma-3,11-dien-2-one
Catalog No.:BCN7607
CAS No.:86917-79-5
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
- (R)-(-)-α-Methylhistamine dihydrobromide
Catalog No.:BCC5665
CAS No.:868698-49-1
- 3',5'-Dimethoxybiphenyl-3-ol
Catalog No.:BCN7529
CAS No.:868666-20-0
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
- Iristectorigenin B
Catalog No.:BCN8391
CAS No.:39012-01-6
- (+)-Puerol B 2''-O-glucoside
Catalog No.:BCN4561
CAS No.:868409-19-2
- Protosappanin A dimethyl acetal
Catalog No.:BCN6517
CAS No.:868405-37-2
- 2,2,5,5-Tetramethylcyclohexane-1,4-dione
Catalog No.:BCN1324
CAS No.:86838-54-2
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- Rhodiosin; Herbacetin-7-O-glucorhamnoside
Catalog No.:BCN8478
CAS No.:86831-54-1
- Neurokinin A (porcine)
Catalog No.:BCC6955
CAS No.:86933-74-6
- Neurokinin B (human, porcine)
Catalog No.:BCC7119
CAS No.:86933-75-7
- AZD8330
Catalog No.:BCC3733
CAS No.:869357-68-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- 14-Deoxy-17-hydroxyandrographolide
Catalog No.:BCN4560
CAS No.:869384-82-7
- Praeroside II
Catalog No.:BCN7001
CAS No.:86940-46-7
- JNJ 10191584 maleate
Catalog No.:BCC7362
CAS No.:869497-75-6
- Tecovirimat
Catalog No.:BCC5518
CAS No.:869572-92-9
- Fmoc-Tyr(tBu)-ol
Catalog No.:BCC2572
CAS No.:86967-51-3
- Obestatin (rat)
Catalog No.:BCC5912
CAS No.:869705-22-6
- A-770041
Catalog No.:BCC1323
CAS No.:869748-10-7
- threo-Guaiacylglycerol beta-coniferyl ether
Catalog No.:BCN1323
CAS No.:869799-76-8
Hic-5 regulates Src-induced invadopodia rosette formation and organization.[Pubmed:30893012]
Mol Biol Cell. 2019 Mar 20:mbcE18100629.
Fibroblasts transformed by the proto-oncogene Src form individual invadopodia that can spontaneously self-organize into large matrix-degrading super-structures called rosettes. However, the mechanisms by which the invadopodia can spatio-temporally reorganize their architecture is not well understood. Herein, we show that Hic-5, a close relative of the scaffold protein paxillin, is essential for the formation and organization of rosettes in active Src-transfected NIH3T3 fibroblasts and cancer-associated fibroblasts. Live cell imaging, combined with domain-mapping analysis of Hic-5 identified critical motifs as well as phosphorylation sites that are required for the formation and dynamics of rosettes. Using pharmacological inhibition and mutant expression, we show that FAK kinase activity along with its proximity to and potential interaction with the LD2,3 motifs of Hic-5 is necessary for rosette formation. Invadopodia dynamics and their coalescence into rosettes was also dependent on Rac1, formin and myosin II activity. Super-resolution microscopy revealed the presence of formin FHOD1 and INF2-mediated unbranched, radial F-actin fibers emanating from invadopodia and rosettes that may facilitate rosette formation. Collectively, our data highlights a novel role for Hic-5 in orchestrating the organization of invadopodia into higher order rosettes which may promote localized matrix degradation necessary for tumor cell invasion. Movie S1 Movie S1 Y527F Src-transfected cell expressing GFP-Hic-5 WT (green) and mCherry-Lifeact (red). Images were acquired every 2 mins for about 2 hours. Movie S2 Movie S2 Y527F Src-transfected cell expressing GFP-Hic-5 DeltaLD2,3 mutant (green) and mCherry-Lifeact (red). Images were acquired every 2 mins for 2 about hours. Movie S3 Movie S3 Y527F Src-transfected cell expressing GFP-Hic-5 WT (green) and mCherry-Lifeact (red) treated with the FAK inhibitor PF-573228 (10muM). Images were acquired every 2 mins for about 2 hours. The FAK inhibitor was added at the time indicated in the video. Movie S4 Movie S4 Y527F Src-transfected cell expressing GFP-Hic-5 WT (green) and mCherry-Lifeact (red) treated with the Rac1 inhibitor NSC23766 (100muM). Images were acquired every 2 mins for about 2 hours. The Rac1 inhibitor was added at the time indicated in the video. Movie S5 Movie S5 Y527F Src-transfected cell expressing GFP-Hic-5 WT (green) and mCherry-Lifeact (red) treated with the ROCK inhibitor Y-27632 (10muM). Images were acquired every 2 mins for about 2 hours. The ROCK inhibitor was added at the time indicated in the video. Movie S6 Movie S6 Y527F Src-transfected cell expressing mCherry-Lifeact (red) treated with the pan formin inhibitor SMIFH2 (5muM). Images were acquired every 1 min for 2 hours. The formin inhibitor was added at the time indicated in the video. Movie S7 Movie S7 Y527F Src-transfected cell expressing mCherry-Lifeact (red) treated with the myosin II inhibitor Blebbistatin (5muM). Images were acquired every 30 seconds for 1 hour. The inhibitor was added at the time indicated in the video.
Characterization of in vitro metabolism of focal adhesion kinase inhibitors by LC/MS/MS.[Pubmed:30807921]
J Pharm Biomed Anal. 2019 May 10;168:163-173.
Focal adhesion kinase (FAK), a non-receptor tyrosine kinase, is critically involved in cell migration, spreading and proliferation at the early step of various cancers. Small molecule inhibitors of FAK are effective to inhibit its activation in the process of tumor formation in cell. To better understand biotransformation of FAK inhibitors, this work has investigated in vitro phase I metabolism of inhibitors (namely PF-573228, PF-562271 and PF-03814735) by rat liver microsomes model. Using liquid chromatography - quadrupole time of flight mass spectrometry and tandem mass spectrometry (LC/Q-TOF/MS and MS/MS), three metabolites of PF-573228 and PF-562271 were observed and characterized, respectively. These in vitro metabolites were reported for the first time. The structures and fragmentation patterns of these metabolites were elucidated, and phase I metabolic pathways for FAK inhibitors were proposed. The main metabolic pathways of PF-573228 were hydroxylation, dehydrogenation and N-dealkylation. For PF-562271, they were hydroxylation and dehydrogenation. Hydroxylation was observed as the primary metabolism for PF-0381473.
Inhibition of FAK Signaling Elicits Lamin A/C-Associated Nuclear Deformity and Cellular Senescence.[Pubmed:30761269]
Front Oncol. 2019 Jan 30;9:22.
Focal adhesion kinase (FAK) is a non-receptor kinase that facilitates tumor aggressiveness. The effects of FAK inhibition include arresting proliferation, limiting metastasis, and inhibiting angiogenesis. PF-573228 is an ATP-competitive inhibitor of FAK. Treating lung cancer cells with PF-573228 resulted in FAK inactivation and changes in the expressions of lamin A/C and nuclear deformity. Since lamin A/C downregulation or deficiency was associated with cellular senescence, the senescence-associated beta-galactosidase (SA-beta-gal) assay was used to investigate whether PF-573228 treatment drove cellular senescence, which showed more SA-beta-gal-positive cells in culture. p53 is known to play a pivotal role in mediating the progression of cellular senescence, and the PF-573228-treated lung cancer cells resulted in a higher p53 expression level. Subsequently, the FAK depletion in lung cancer cells was employed to confirm the role of FAK inhibition on cellular senescence. FAK depletion and pharmacological inhibition of lung cancer cells elicited similar patterns of cellular senescence, lamin A/C downregulation, and p53 upregulation, implying that FAK signaling is associated with the expression of p53 and the maintenance of lamin A/C levels to shape regular nuclear morphology and manage anti-senescence. Conversely, FAK inactivation led to p53 upregulation, disorganization of the nuclear matrix, and consequently cellular senescence. Our data suggest a new FAK signaling pathway, in that abolishing FAK signaling can activate the senescence program in cells. Triggering cellular senescence could be a new therapeutic approach to limit tumor growth.
Extremely low frequency electromagnetic fields promote mesenchymal stem cell migration by increasing intracellular Ca(2+) and activating the FAK/Rho GTPases signaling pathways in vitro.[Pubmed:29784011]
Stem Cell Res Ther. 2018 May 21;9(1):143.
BACKGROUND: The ability of mesenchymal stem cells (MSCs) to migrate to the desired tissues or lesions is crucial for stem cell-based regenerative medicine and tissue engineering. Optimal therapeutics for promoting MSC migration are expected to become an effective means for tissue regeneration. Electromagnetic fields (EMF), as a noninvasive therapy, can cause a lot of biological changes in MSCs. However, whether EMF can promote MSC migration has not yet been reported. METHODS: We evaluated the effects of EMF on cell migration in human bone marrow-derived MSCs. With the use of Helmholtz coils and an EMF stimulator, 7.5, 15, 30, 50, and 70 Hz/1 mT EMF was generated. Additionally, we employed the L-type calcium channel blocker verapamil and the focal adhesion kinase (FAK) inhibitor PF-573228 to investigate the role of intracellular calcium content, cell adhesion proteins, and the Rho GTPase protein family (RhoA, Rac1, and Cdc42) in EMF-mediated MSC migration. Cell adhesion proteins (FAK, talin, and vinculin) were detected by Western blot analysis. The Rho GTPase protein family activities were assessed by G-LISA, and F-actin levels, which reflect actin cytoskeletal organization, were detected using immunofluorescence. RESULTS: All the 7.5, 15, 30, 50, and 70 Hz/1 mT EMF promoted MSC migration. EMF increased MSC migration in an intracellular calcium-dependent manner. Notably, EMF-enhanced migration was mediated by FAK activation, which was critical for the formation of focal contacts, as evidenced by increased talin and vinculin expression. Moreover, RhoA, Rac1, and Cdc42 were activated by FAK to increase cytoskeletal organization, thus promoting cell contraction. CONCLUSIONS: EMF promoted MSC migration by increasing intracellular calcium and activating the FAK/Rho GTPase signaling pathways. This study provides insights into the mechanisms of MSC migration and will enable the rational design of targeted therapies to improve MSC engraftment.
Inhibiting focal adhesion kinase (FAK) blocks IL-4 induced VCAM-1 expression and eosinophil recruitment in vitro and in vivo.[Pubmed:29633338]
J Leukoc Biol. 2018 Jul;104(1):147-158.
Leukocyte recruitment plays a critical role during both normal inflammation and chronic inflammatory diseases, and ongoing studies endeavor to better understand the complexities of this process. Focal adhesion kinase (FAK) is well known for its role in cancer, yet it also has been shown to regulate aspects of neutrophil and B16 melanoma cell recruitment by rapidly influencing endothelial cell focal adhesion dynamics and junctional opening. Recently, we found that FAK related non-kinase (FRNK), a protein that is often used as a FAK dominant negative, blocked eosinophil transmigration by preventing the transcription of vascular cell adhesion molecule-1 (VCAM-1) and eotaxin-3 (CCL26). Surprisingly, the blocking occurred even in the absence of endogenous FAK. To better understand the role of FAK in leukocyte recruitment, we used a FAK-specific inhibitor (PF-573228) and determined the effect on IL-4 induced eosinophil recruitment in vitro and in vivo. PF-573228 prevented the expression of VCAM-1 and CCL26 expression in IL-4-stimulated human endothelial cells in vitro. As a result, eosinophil adhesion and transmigration were blocked. PF-572338 also prevented IL-4-induced VCAM-1 expression in vivo. Using brightfield intravital microscopy, we found that PF-573228 decreased leukocyte rolling flux, adhesion, and emigration. We specifically examined eosinophil recruitment in vivo by using an eosinophil-GFP reporter mouse and found PF-573228 attenuated eosinophil emigration. This study reveals that a FAK inhibitor influences inflammation through its action on eosinophil recruitment.
Focal adhesion kinase a potential therapeutic target for pancreatic cancer and malignant pleural mesothelioma.[Pubmed:29303405]
Cancer Biol Ther. 2018 Apr 3;19(4):316-327.
The non-receptor cytoplasmic tyrosine kinase, Focal Adhesion Kinase (FAK) is known to play a key role in a variety of normal and cancer cellular functions such as survival, proliferation, migration and invasion. It is highly active and overexpressed in various cancers including Pancreatic Ductal Adenocarcinoma (PDAC) and Malignant Pleural Mesothelioma (MPM). Here, initially, we demonstrate that FAK is overexpressed in both PDAC and MPM cell lines. Then we analyze effects of two small molecule inhibitors PF-573228, and PF-431396, which are dual specificity inhibitors of FAK and proline rich tyrosine kinase 2 (PYK2), as well as VS-6063, another small molecule inhibitor that specifically inhibits FAK but not PYK2 for cell growth, motility and invasion of PDAC and MPM cell lines. Treatment with PF-573228, PF-431396 and VS-6063 cells resulted in a dose-dependent inhibition of growth and anchorage-independent colony formation in both cancer cell lines. Furthermore, these compounds suppressed the phosphorylation of FAK at its active site, Y397, and functionally induced significant apoptosis and cell cycle arrest in both cell lines. Using the ECIS (Electric cell-substrate impedance sensing) system, we found that treatment of both PF compounds suppressed adherence and migration of PDAC cells on fibronectin. Interestingly, 3D-tumor organoids derived from autochthonous KC (Kras;PdxCre) mice treated with PF-573228 revealed a significant decrease in tumor organoid size and increase in organoid cell death. Taken together, our results show that FAK is an important target for mesothelioma and pancreatic cancer therapy that merit further translational studies.
Multiple intracellular signaling pathways orchestrate adipocytic differentiation of human bone marrow stromal stem cells.[Pubmed:29298881]
Biosci Rep. 2018 Jan 30;38(1). pii: BSR20171252.
Bone marrow adipocyte formation plays a role in bone homeostasis and whole body energy metabolism. However, the transcriptional landscape and signaling pathways associated with adipocyte lineage commitment and maturation are not fully delineated. Thus, we performed global gene expression profiling during adipocyte differentiation of human bone marrow stromal (mesenchymal) stem cells (hMSCs) and identified 2,589 up-regulated and 2,583 down-regulated mRNA transcripts. Pathway analysis on the up-regulated gene list untraveled enrichment in multiple signaling pathways including insulin receptor signaling, focal Adhesion, metapathway biotransformation, a number of metabolic pathways e.g. selenium metabolism, Benzo(a)pyrene metabolism, fatty acid, triacylglycerol, ketone body metabolism, tryptophan metabolism, and catalytic cycle of mammalian flavin-containing monooxygenase (FMOs). On the other hand, pathway analysis on the down-regulated genes revealed significant enrichment in pathways related to cell cycle regulation. Based on these data, we assessed the effect of pharmacological inhibition of FAK signaling using PF-573228, PF-562271, and InsR/IGF-1R using NVP-AEW541 and GSK-1904529A on adipocyte differentiation. hMSCs exposed to FAK or IGF-1R/InsR inhibitors exhibited fewer adipocyte formation (27-58% inhibition, P<0005). Concordantly, the expression of adipocyte-specific genes AP2, AdipoQ, and CEBPalpha was significantly reduced. On the other hand, we did not detect significant effects on cell viability as a result of FAK or IGF-1R/InsR inhibition. Our data identified FAK and insulin signaling as important intracellular signaling pathways relevant to bone marrow adipogenesis.
FUS-CHOP Promotes Invasion in Myxoid Liposarcoma through a SRC/FAK/RHO/ROCK-Dependent Pathway.[Pubmed:29190494]
Neoplasia. 2018 Jan;20(1):44-56.
Deregulated SRC/FAK signaling leads to enhanced migration and invasion in many types of tumors. In myxoid and round cell liposarcoma (MRCLS), an adipocytic tumor characterized by the expression of the fusion oncogene FUS-CHOP, SRC have been found as one of the most activated kinases. Here we used a cell-of-origin model of MRCLS and an MRCLS cell line to thoroughly characterize the mechanisms of cell invasion induced by FUS-CHOP using in vitro (3D spheroid invasion assays) and in vivo (chicken chorioallantoic membrane model) approaches. FUS-CHOP expression activated SRC-FAK signaling and increased the invasive ability of MRCLS cells. In addition, FAK expression was found to significantly correlate with tumor aggressiveness in sarcoma patient samples. The involvement of SRC/FAK activation in FUS-CHOP-mediated invasion was further confirmed using the SRC inhibitor dasatinib, the specific FAK inhibitor PF-573228, and FAK siRNA. Notably, dasatinib and PF573228 could also efficiently block the invasion of cancer stem cell subpopulations. Downstream of SRC/FAK signaling, we found that FUS-CHOP expression increases the levels of the RHO/ROCK downstream effector phospho-MLC2 (T18/S19) and that this activation was prevented by dasatinib or PF573228. Moreover, the ROCK inhibitor RKI-1447 was able to completely abolish invasion in FUS-CHOP-expressing cells. These data uncover the involvement of SRC/FAK/RHO/ROCK signaling axis in FUS-CHOP-mediated invasion, thus providing a rationale for testing inhibitors of this pathway as potential novel antimetastatic agents for MRCLS treatment.
Anti-invasive effects of CXCR4 and FAK inhibitors in non-small cell lung carcinomas with mutually inactivated p53 and PTEN tumor suppressors.[Pubmed:28733702]
Invest New Drugs. 2017 Dec;35(6):718-732.
Non-small cell lung carcinoma (NSCLC) is the most common type of lung cancer. At the time of diagnosis, a large percentage of NSCLC patients have already developed metastasis, responsible for extremely high mortality rates. CXCR4 receptor and focal adhesion kinase (FAK) are known to regulate such invasive cancer behavior. Their expression is downregulated by p53 and PTEN tumor suppressors which are commonly co-inactivated in NSCLC patients and contribute to metastasis. Therefore, targeting CXCR4 or FAK seems to be a promising strategy in suppressing metastatic spread of p53/PTEN deficient NSCLCs. In this study, we first examined the invasive characteristics of NSCLC cells with suppressed p53 and PTEN activity using wound healing, gelatin degradation and invasion assays. Further, changes in the expression of CXCR4 and FAK were evaluated by RT-qPCR and Western Blot analysis. Finally, we tested the ability of CXCR4 and FAK inhibitors (WZ811 and PF-573228, respectively) to suppress the migratory and invasive potential of p53/PTEN deficient NSCLC cells, in vitro and in vivo using metastatic models of human NSCLC. Our results showed that cells with mutually inactive p53 and PTEN have significantly increased invasive potential associated with hyperactivation of CXCR4 and FAK signaling pathways. Treatments with WZ811 and PF-573228 inhibitors significantly reduced migratory and invasive capacity in vitro and showed a trend of improved survival in vivo. Accordingly, we demonstrated that p53/PTEN deficient NSCLCs have extremely invasive phenotype and provided a rationale for the use of CXCR4 or FAK inhibitors for the suppression of NSCLC dissemination.
Ang II-AT2R increases mesenchymal stem cell migration by signaling through the FAK and RhoA/Cdc42 pathways in vitro.[Pubmed:28697804]
Stem Cell Res Ther. 2017 Jul 12;8(1):164.
BACKGROUND: Mesenchymal stem cells (MSCs) migrate via the bloodstream to sites of injury and are possibly attracted by inflammatory factors. As a proinflammatory mediator, angiotensin II (Ang II) reportedly enhances the migration of various cell types by signaling via the Ang II receptor in vitro. However, few studies have focused on the effects of Ang II on MSC migration and the underlying mechanisms. METHODS: Human bone marrow MSCs migration was measured using wound healing and Boyden chamber migration assays after treatments with different concentrations of Ang II, an AT1R antagonist (Losartan), and/or an AT2R antagonist (PD-123319). To exclude the effect of proliferation on MSC migration, we measured MSC proliferation after stimulation with the same concentration of Ang II. Additionally, we employed the focal adhesion kinase (FAK) inhibitor PF-573228, RhoA inhibitor C3 transferase, Rac1 inhibitor NSC23766, or Cdc42 inhibitor ML141 to investigate the role of cell adhesion proteins and the Rho-GTPase protein family (RhoA, Rac1, and Cdc42) in Ang II-mediated MSC migration. Cell adhesion proteins (FAK, Talin, and Vinculin) were detected by western blot analysis. The Rho-GTPase family protein activities were assessed by G-LISA and F-actin levels, which reflect actin cytoskeletal organization, were detected by using immunofluorescence. RESULTS: Human bone marrow MSCs constitutively expressed AT1R and AT2R. Additionally, Ang II increased MSC migration in an AT2R-dependent manner. Notably, Ang II-enhanced migration was not mediated by Ang II-mediated cell proliferation. Interestingly, Ang II-enhanced migration was mediated by FAK activation, which was critical for the formation of focal contacts, as evidenced by increased Talin and Vinculin expression. Moreover, RhoA and Cdc42 were activated by FAK to increase cytoskeletal organization, thus promoting cell contraction. Furthermore, FAK, Talin, and Vinculin activation and F-actin reorganization in response to Ang II were prevented by PD-123319 but not Losartan, indicating that FAK activation and F-actin reorganization were downstream of AT2R. CONCLUSIONS: These data indicate that Ang II-AT2R regulates human bone marrow MSC migration by signaling through the FAK and RhoA/Cdc42 pathways. This study provides insights into the mechanisms by which MSCs home to injury sites and will enable the rational design of targeted therapies to improve MSC engraftment.
FAK-heterozygous mice display enhanced tumour angiogenesis.[Pubmed:23799510]
Nat Commun. 2013;4:2020.
Genetic ablation of endothelial focal adhesion kinase (FAK) can inhibit pathological angiogenesis, suggesting that loss of endothelial FAK is sufficient to reduce neovascularization. Here we show that reduced stromal FAK expression in FAK-heterozygous mice unexpectedly enhances both B16F0 and CMT19T tumour growth and angiogenesis. We further demonstrate that cell proliferation and microvessel sprouting, but not migration, are increased in serum-stimulated FAK-heterozygous endothelial cells. FAK-heterozygous endothelial cells display an imbalance in FAK phosphorylation at pY397 and pY861 without changes in Pyk2 or Erk1/2 activity. By contrast, serum-stimulated phosphorylation of Akt is enhanced in FAK-heterozygous endothelial cells and these cells are more sensitive to Akt inhibition. Additionally, low doses of a pharmacological FAK inhibitor, although too low to affect FAK autophosphorylation in vitro, can enhance angiogenesis ex vivo and tumour growth in vivo. Our results highlight a potential novel role for FAK as a nonlinear, dose-dependent regulator of angiogenesis where heterozygous levels of FAK enhance angiogenesis.
Cellular characterization of a novel focal adhesion kinase inhibitor.[Pubmed:17395594]
J Biol Chem. 2007 May 18;282(20):14845-52.
Focal adhesion kinase (FAK) is a member of a family of non-receptor protein-tyrosine kinases that regulates integrin and growth factor signaling pathways involved in cell migration, proliferation, and survival. FAK expression is increased in many cancers, including breast and prostate cancer. Here we describe perturbation of adhesion-mediated signaling with a FAK inhibitor, PF-573,228. In vitro, this compound inhibited purified recombinant catalytic fragment of FAK with an IC(50) of 4 nM. In cultured cells, PF-573,228 inhibited FAK phosphorylation on Tyr(397) with an IC(50) of 30-100 nM. Treatment of cells with concentrations of PF-573,228 that significantly decreased FAK Tyr(397) phosphorylation failed to inhibit cell growth or induce apoptosis. In contrast, treatment with PF-573,228 inhibited both chemotactic and haptotactic migration concomitant with the inhibition of focal adhesion turnover. These studies show that PF-573,228 serves as a useful tool to dissect the functions of FAK in integrin-dependent signaling pathways in normal and cancer cells and forms the basis for the generation of compounds amenable for preclinical and patient trials.


