PF-562271ATP-competitive FAK inhibitor, reversible CAS# 717907-75-0 |
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- CEP-37440
Catalog No.:BCC5145
CAS No.:1391712-60-9
- PF-431396
Catalog No.:BCC3971
CAS No.:717906-29-1
- TAE226 (NVP-TAE226)
Catalog No.:BCC3885
CAS No.:761437-28-9
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 717907-75-0 | SDF | Download SDF |
| PubChem ID | 11713159 | Appearance | Powder |
| Formula | C21H20F3N7O3S | M.Wt | 507.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 48 mg/mL (94.58 mM) *"≥" means soluble, but saturation unknown. | ||
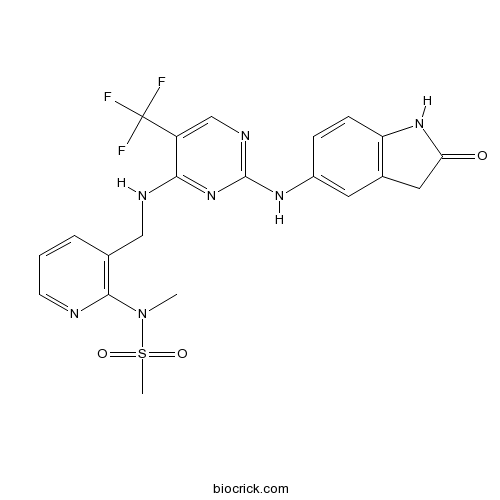
| Chemical Name | N-methyl-N-[3-[[[2-[(2-oxo-1,3-dihydroindol-5-yl)amino]-5-(trifluoromethyl)pyrimidin-4-yl]amino]methyl]pyridin-2-yl]methanesulfonamide | ||
| SMILES | CN(C1=C(C=CC=N1)CNC2=NC(=NC=C2C(F)(F)F)NC3=CC4=C(C=C3)NC(=O)C4)S(=O)(=O)C | ||
| Standard InChIKey | MZDKLVOWGIOKTN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PF-562271 is a potent, ATP-competitive, reversible inhibitor of FAK with IC50 of 1.5 nM, ~10-fold less potent for Pyk2 than FAK and >100-fold selectivity against other protein kinases, except for some CDKs. | |||||
| Targets | FAK | Pyk2 | ||||
| IC50 | 1.5 nM | 14 nM | ||||

PF-562271 Dilution Calculator

PF-562271 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9705 mL | 9.8524 mL | 19.7048 mL | 39.4096 mL | 49.2621 mL |
| 5 mM | 0.3941 mL | 1.9705 mL | 3.941 mL | 7.8819 mL | 9.8524 mL |
| 10 mM | 0.197 mL | 0.9852 mL | 1.9705 mL | 3.941 mL | 4.9262 mL |
| 50 mM | 0.0394 mL | 0.197 mL | 0.3941 mL | 0.7882 mL | 0.9852 mL |
| 100 mM | 0.0197 mL | 0.0985 mL | 0.197 mL | 0.3941 mL | 0.4926 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF-562271 is a potent, ATP-competitive and reversible inhibitor of both focal adhesion kinase (FAK), a non-receptor tyrosine kinase involved in a variety of cellular events, and proline-rich tyrosine kinase 2 (Pyk2), an FAK homolog containing 48% amino acid identity, with half maximal inhibitory concentration (IC50) of 1.5 nmol/L and 14 nmol/L respectively. As a potential therapeutic agent either alone or in combination with other agents for the treatment of cancer, PF-562271 has been reported to effectively inhibit the proliferation of tumors in both xenograft and transgenic mouse models, in which it dose-dependently inhibits FAK phosphorylation in tumor-bearing mice with half maximal effective concentration (EC50) of 93 ng/mL.
References:
[1]Stokes JB, Adair SJ, Slack-Davis JK, Walters DM, Tilghman RW, Hershey ED, Lowrey B, Thomas KS, Bouton AH, Hwang RF, Stelow EB, Parsons JT, Bauer TW. Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther. 2011 Nov;10(11):2135-45. doi: 10.1158/1535-7163.MCT-11-0261. Epub 2011 Sep 8.
[2]Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, Wessel M, Marr E, Griffor M, Vajdos F. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008 Mar 15;68(6):1935-44. doi: 10.1158/0008-5472.CAN-07-5155.
- PF-431396
Catalog No.:BCC3971
CAS No.:717906-29-1
- Verbenacine
Catalog No.:BCN6473
CAS No.:717901-03-6
- Vidofludimus
Catalog No.:BCC5387
CAS No.:717824-30-1
- Clazamycin B
Catalog No.:BCN1961
CAS No.:71774-49-7
- Nafcillin Sodium
Catalog No.:BCC4805
CAS No.:7177-50-6
- Ampicillin Trihydrate
Catalog No.:BCC8820
CAS No.:7177-48-2
- Avermectin B1
Catalog No.:BCC1381
CAS No.:71751-41-2
- Tilbroquinol
Catalog No.:BCC4033
CAS No.:7175-09-9
- Vasicine Hydrochloride
Catalog No.:BCC8265
CAS No.:7174-27-8
- Chilenine
Catalog No.:BCN7799
CAS No.:71700-15-7
- Amisulpride
Catalog No.:BCC4459
CAS No.:71675-85-9
- H-His-NH2.2HCl
Catalog No.:BCC2955
CAS No.:71666-95-0
- Lys-[Des-Arg9]Bradykinin
Catalog No.:BCC5991
CAS No.:71800-36-7
- Clazamycin A
Catalog No.:BCN1960
CAS No.:71806-55-8
- H-D-Ala-NH2.HCl
Catalog No.:BCC3197
CAS No.:71810-97-4
- Z-Thr-ol
Catalog No.:BCC2574
CAS No.:71811-27-3
- Demethoxyencecalinol
Catalog No.:BCN7765
CAS No.:71822-00-9
- Ivermectin B1a
Catalog No.:BCC9005
CAS No.:71827-03-7
- Sativan
Catalog No.:BCN6815
CAS No.:71831-00-0
- Borrelidin
Catalog No.:BCC7964
CAS No.:7184-60-3
- Camaldulenic acid
Catalog No.:BCN3928
CAS No.:71850-15-2
- PHA-793887
Catalog No.:BCC2521
CAS No.:718630-59-2
- 2-Amino-5-chlorobenzophenone
Catalog No.:BCC8535
CAS No.:719-59-5
- S-Adenosyl-L-methionine tosylate
Catalog No.:BCN2230
CAS No.:71914-80-2
Involvement of FAK-ERK2 signaling pathway in CKAP2-induced proliferation and motility in cervical carcinoma cell lines.[Pubmed:28522860]
Sci Rep. 2017 May 18;7(1):2117.
Cervical carcinoma is the fourth most common cause of death in woman, caused by human papillomavirus (HPV) infections and arising from the cervix. Cytoskeleton-associated protein 2 (CKAP2), also known as tumor-associated microtubule-associated protein, has been linked to tumorigenic effects. In the present study, we screened CKAP2 as a new candidate gene which promotes development of cervical carcinoma, in two independent datasets (TCGA and GSE27678). Results showed that CKAP2 expression was significantly up-regulated in cervical cancerous tissues compared with normal counterparts. Gene set enrichment analysis (GSEA) showed that metastasis, cell cycle and FAK pathways were related with elevated CKAP2 expression. Knockdown of CKAP2 expression significantly inhibited cell proliferation, migration and invasion both in HeLa and C-33A cells. And depletion of CKAP2 down-regulated the expression of metastasis and cell cycle related proteins as well as the phosphorylation of ERK2 (p-ERK2), except E-cadherin. In vivo experiment revealed that knockdown of CKAP2 inhibited C-33A cells proliferation. However, FAK inhibitor PF-562271 and ERK2 inhibitor VX-11e treatment significantly inhibited CKAP2 overexpression-induced cell proliferation, migration and invasion in SiHa cells. In conclusion, our study suggests that CKAP2 acts as a functional oncogene in cervical carcinoma development and may exert its function by targeting FAK-ERK2 signaling pathway.
SphK1 modulates cell migration and EMT-related marker expression by regulating the expression of p-FAK in colorectal cancer cells.[Pubmed:28405684]
Int J Mol Med. 2017 May;39(5):1277-1284.
Sphingosine kinase 1 (SphK1) plays an important role in colorectal carcinoma metastasis. However, whether SphK1 modulates epithelial-mesenchymal transition (EMT)-related marker expression and the underlying mechanisms remain unclear. In this study, in order to clarify this issue, we used various colorectal cancer (CRC) cell lines, Caco2, HT29, RKO and HCT116. Each of the cell lines was divided into 3 groups as follows: the control group, SKI- (SphK1 inhibitor) group and PF-562271 [focal adhesion kinase (FAK) inhibitor] group. The migratory ability of the cells was examined by Transwell chamber assay. The mRNA and protein expression levels of SphK1, FAK (p-FAK), Slug, vimentin, N-cadherin and E-cadherin were detected by PCR and western blot analysis, respectively. The results revealed that the suppression of SphK1 reduced the cell migratory ability, and decreased the expression of Slug, vimentin and N-cadherin; however, the expression of E-cadherin was increased. Moreover, the inhibition of SphK1 reduced the expression of p-FAK. The inhibition of FAK (p-FAK) also decreased the cell migratory ability, and decreased the expression of Slug, vimentin and N-cadherin, whereas the expression of E-cadherin was increased. Thus, our data suggest that SphK1 modulates the expression of EMT-related markers and cell migration by regulating the expression of p-FAK in CRC cells. Thus, SphK1 may play a functional role in mediating the EMT process in CRC.
miR-193b-Regulated Signaling Networks Serve as Tumor Suppressors in Liposarcoma and Promote Adipogenesis in Adipose-Derived Stem Cells.[Pubmed:28882999]
Cancer Res. 2017 Nov 1;77(21):5728-5740.
Well-differentiated and dedifferentiated liposarcomas (WDLS/DDLS) account for approximately 13% of all soft tissue sarcoma in adults and cause substantial morbidity or mortality in the majority of patients. In this study, we evaluated the functions of miRNA (miR-193b) in liposarcoma in vitro and in vivo Deep RNA sequencing on 93 WDLS, 145 DDLS, and 12 normal fat samples demonstrated that miR-193b was significantly underexpressed in DDLS compared with normal fat. Reintroduction of miR-193b induced apoptosis in liposarcoma cells and promoted adipogenesis in human adipose-derived stem cells (ASC). Integrative transcriptomic and proteomic analysis of miR-193b-target networks identified novel direct targets, including CRK-like proto-oncogene (CRKL) and focal adhesion kinase (FAK). miR-193b was found to regulate FAK-SRC-CRKL signaling through CRKL and FAK. miR-193b also stimulated reactive oxygen species signaling by targeting the antioxidant methionine sulfoxide reductase A to modulate liposarcoma cell survival and ASC differentiation state. Expression of miR-193b in liposarcoma cells was downregulated by promoter methylation, resulting at least in part from increased expression of the DNA methyltransferase DNMT1 in WDLS/DDLS. In vivo, miR-193b mimetics and FAK inhibitor (PF-562271) each inhibited liposarcoma xenograft growth. In summary, miR-193b not only functions as a tumor suppressor in liposarcoma but also promotes adipogenesis in ASC. Furthermore, this study reveals key tyrosine kinase and DNA methylation pathways in liposarcoma, some with immediate implications for therapeutic exploration. Cancer Res; 77(21); 5728-40. (c)2017 AACR.
Focal adhesion kinase signaling regulates anti-inflammatory function of bone marrow mesenchymal stromal cells induced by biomechanical force.[Pubmed:28647573]
Cell Signal. 2017 Oct;38:1-9.
Mesenchymal stromal cells (MSCs) have tremendous potential for use in regenerative medicine due to their multipotency and immune cell regulatory functions. Biomimetic physical forces have been shown to direct differentiation and maturation of MSCs in tissue engineering applications; however, the effect of force on immunomodulatory activity of MSCs has been largely overlooked. Here we show in human bone marrow-derived MSCs that wall shear stress (WSS) equivalent to the fluid frictional force present in the adult arterial vasculature significantly enhances expression of four genes that mediate MSC immune regulatory function, PTGS2, HMOX1, IL1RN, and TNFAIP6. Several mechanotransduction pathways are stimulated by WSS, including calcium ion (Ca(2+)) flux and activation of Akt, MAPK, and focal adhesion kinase (FAK). Inhibition of PI3K-Akt by LY294002 or Ca(2+) signaling with chelators, ion channel inhibitors, or Ca(2+) free culture conditions failed to attenuate WSS-induced COX2 expression. In contrast, the FAK inhibitor PF-562271 blocked COX2 induction, implicating focal adhesions as critical sensory components upstream of this key immunomodulatory factor. In co-culture assays, WSS preconditioning stimulates MSC anti-inflammatory activity to more potently suppress TNF-alpha production by activated immune cells, and this improved potency depended upon the ability of FAK to stimulate COX2 induction. Taken together, our data demonstrate that biomechanical force potentiates the reparative and regenerative properties of MSCs through a FAK signaling cascade and highlights the potential for innovative force-based approaches for enhancement in MSC therapeutic efficacy.
Control of long-distance cell-to-cell communication and autophagosome transfer in squamous cell carcinoma via tunneling nanotubes.[Pubmed:28423494]
Oncotarget. 2017 Mar 28;8(13):20939-20960.
Tunneling nanotubes (TnTs) are thin channels that temporally connect nearby cells allowing the cell-to-cell trafficking of biomolecules and organelles. The presence or absence of TnTs in human neoplasms and the mechanisms of TnT assembly remains largely unexplored. In this study, we have identified TnTs in tumor cells derived from squamous cell carcinomas (SCC) cultured under bi-dimensional and tri-dimensional conditions and also in human SCC tissues. Our study demonstrates that TnTs are not specific of epithelial or mesenchymal phenotypes and allow the trafficking of endosomal/lysosomal vesicles, mitochondria, and autophagosomes between both types of cells. We have identified focal adhesion kinase (FAK) as a key molecule required for TnT assembly via a mechanism involving the MMP-2 metalloprotease. We have also found that the FAK inhibitor PF-562271, which is currently in clinical development for cancer treatment, impairs TnT formation. Finally, FAK-deficient cells transfer lysosomes/autophagosomes to FAK-proficient cells via TnTs which may represent a novel mechanism to adapt to the stress elicited by impaired FAK signaling. Collectively, our results strongly suggest a link between FAK, MMP-2, and TnT, and unveil new vulnerabilities that can be exploited to efficiently eradicate cancer cells.


