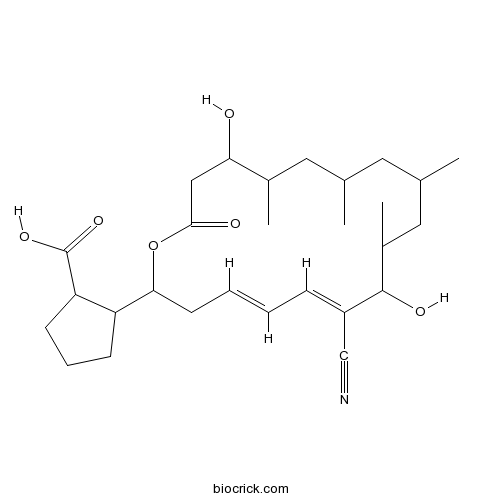
BorrelidinCAS# 7184-60-3 |
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- Simeprevir
Catalog No.:BCC1949
CAS No.:923604-59-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7184-60-3 | SDF | Download SDF |
| PubChem ID | 5357974 | Appearance | Powder |
| Formula | C28H43NO6 | M.Wt | 489.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Ethanol : ≥ 100 mg/mL (204.23 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-[(4E,6E)-7-cyano-8,16-dihydroxy-9,11,13,15-tetramethyl-18-oxo-1-oxacyclooctadeca-4,6-dien-2-yl]cyclopentane-1-carboxylic acid | ||
| SMILES | CC1CC(CC(C(C(=CC=CCC(OC(=O)CC(C(C1)C)O)C2CCCC2C(=O)O)C#N)O)C)C | ||
| Standard InChIKey | OJCKRNPLOZHAOU-SLNPHPKOSA-N | ||
| Standard InChI | InChI=1S/C28H43NO6/c1-17-12-18(2)14-20(4)27(32)21(16-29)8-5-6-11-25(22-9-7-10-23(22)28(33)34)35-26(31)15-24(30)19(3)13-17/h5-6,8,17-20,22-25,27,30,32H,7,9-15H2,1-4H3,(H,33,34)/b6-5+,21-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antibiotic and antiangiogenic. Selectively inhibits threonyl-tRNA synthetase (ThrRS). Exhibits antiangiogenic activity in a mouse model of tumor angiogenesis. Induces apoptosis in capillary tube-forming endothelial cells; disrupts capillary tubes and inhibits their formation (IC50 = 0.8 nM in rat aorta). Induces the unfolded protein response (UPR) and apoptosis in oral cancer cells. |

Borrelidin Dilution Calculator

Borrelidin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0423 mL | 10.2116 mL | 20.4232 mL | 40.8463 mL | 51.0579 mL |
| 5 mM | 0.4085 mL | 2.0423 mL | 4.0846 mL | 8.1693 mL | 10.2116 mL |
| 10 mM | 0.2042 mL | 1.0212 mL | 2.0423 mL | 4.0846 mL | 5.1058 mL |
| 50 mM | 0.0408 mL | 0.2042 mL | 0.4085 mL | 0.8169 mL | 1.0212 mL |
| 100 mM | 0.0204 mL | 0.1021 mL | 0.2042 mL | 0.4085 mL | 0.5106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sativan
Catalog No.:BCN6815
CAS No.:71831-00-0
- Ivermectin B1a
Catalog No.:BCC9005
CAS No.:71827-03-7
- Demethoxyencecalinol
Catalog No.:BCN7765
CAS No.:71822-00-9
- Z-Thr-ol
Catalog No.:BCC2574
CAS No.:71811-27-3
- H-D-Ala-NH2.HCl
Catalog No.:BCC3197
CAS No.:71810-97-4
- Clazamycin A
Catalog No.:BCN1960
CAS No.:71806-55-8
- Lys-[Des-Arg9]Bradykinin
Catalog No.:BCC5991
CAS No.:71800-36-7
- PF-562271
Catalog No.:BCC3674
CAS No.:717907-75-0
- PF-431396
Catalog No.:BCC3971
CAS No.:717906-29-1
- Verbenacine
Catalog No.:BCN6473
CAS No.:717901-03-6
- Vidofludimus
Catalog No.:BCC5387
CAS No.:717824-30-1
- Clazamycin B
Catalog No.:BCN1961
CAS No.:71774-49-7
- Camaldulenic acid
Catalog No.:BCN3928
CAS No.:71850-15-2
- PHA-793887
Catalog No.:BCC2521
CAS No.:718630-59-2
- 2-Amino-5-chlorobenzophenone
Catalog No.:BCC8535
CAS No.:719-59-5
- S-Adenosyl-L-methionine tosylate
Catalog No.:BCN2230
CAS No.:71914-80-2
- ProINDY
Catalog No.:BCC6350
CAS No.:719277-30-2
- Sarmentosin
Catalog No.:BCN4275
CAS No.:71933-54-5
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
- Leiocarposide
Catalog No.:BCC8196
CAS No.:71953-77-0
- Artemether
Catalog No.:BCN5973
CAS No.:71963-77-4
- Fmoc-Asp(OtBu)-OH
Catalog No.:BCC3469
CAS No.:71989-14-5
- Fmoc-Asn-OH
Catalog No.:BCC3079
CAS No.:71989-16-7
- Fmoc-Glu(OtBu)-OH
Catalog No.:BCC3494
CAS No.:71989-18-9
Chemoselective enrichment as a tool to increase access to bioactive natural products: Case study borrelidin.[Pubmed:26255540]
Bioorg Med Chem Lett. 2015 Nov 1;25(21):4767-4769.
Chemoselective purification technologies have seen great success in biomolecule isolation, with a classic example being the genetically-encoded His tag utilized to enrich desired proteins from a crude lysate. We sought to translate this purification tactic into a powerful tool for the isolation of natural products and demonstrate that chemoselective enrichment can reduce the number of purification steps required and increase the yield obtained for important natural products, as compared to the use of traditional chromatography methods alone. To date, we have reported reversible enrichment tags for three functional groups, carboxylic acids and aliphatic or aryl hydroxyls. To illustrate the power of chemoselectivity-mediated purification of natural products, we present here an improved isolation of Borrelidin. Application of our carboxylic acid tag yielded pure Borrelidin in only two steps and with double the yield acquired with traditional chromatography methods. These results highlight the utility of this orthogonal strategy to facilitate the isolation of natural products, which are often present in minute quantities in their source materials.
Borrelidin Induces the Unfolded Protein Response in Oral Cancer Cells and Chop-Dependent Apoptosis.[Pubmed:26617965]
ACS Med Chem Lett. 2015 Sep 8;6(11):1122-7.
Oral squamous cell carcinoma (OSCC) is the most common cancer affecting the oral cavity, and US clinics will register about 30,000 new patients in 2015. Current treatment modalities include chemotherapy, surgery, and radiotherapy, which often result in astonishing disfigurement. Cancers of the head and neck display enhanced levels of glucose-regulated proteins and translation initiation factors associated with endoplasmic reticulum (ER) stress and the unfolded protein response (UPR). Previous work demonstrated that chemically enforced UPR could overwhelm these adaptive features and selectively kill malignant cells. The threonyl-tRNA synthetase (ThRS) inhibitor Borrelidin and two congeners were discovered in a cell-based chemical genomic screen. Borrelidin increased XBP1 splicing and led to accumulation of phosphorylated eIF2alpha and UPR-associated genes, prior to death in panel of OSCC cells. Murine embryonic fibroblasts (MEFs) null for GCN2 and PERK were less able to accumulate UPR markers and were resistant to Borrelidin. This study demonstrates that UPR induction is a feature of ThRS inhibition and adds to a growing body of literature suggesting ThRS inhibitors might selectively target cancer cells.
Borrelidin Isolated from Streptomyces sp. Inhibited Adipocyte Differentiation in 3T3-L1 Cells via Several Factors Including GATA-Binding Protein 3.[Pubmed:26424016]
Biol Pharm Bull. 2015;38(10):1504-11.
An inhibitor of 3T3-L1 adipocyte differentiation was isolated from Streptomyces sp. TK08330 and identified by spectroscopy as the 18-membered macrolide Borrelidin. Treatment with 1.0 muM Borrelidin suppressed intracellular lipid accumulation by 80% and inhibited the expression of adipocyte-specific genes. Borrelidin suppressed the mRNA expression of two master regulators of adipocyte differentiation, peroxisome proliferator-activated receptor gamma (PPARgamma) and CCAAT/enhancer binding protein (C/EBPalpha). Studies on well-known upstream regulators of PPARgamma revealed that Borrelidin down-regulated C/EBPdelta mRNA expression but did not affect expression of C/EBPbeta. Borrelidin increased mRNA expression of negative regulators of differentiation such as GATA-binding protein (GATA) 3, Kruppel-like factor (KLF) 3 and KLF7, as well as positive regulators, KLF4, KLF6 and KLF15, at early stages of differentiation. To elucidate a primary mediator of Borrelidin differentiation inhibitory activity, small interfering RNA (siRNA) transfection experiments were performed. The mRNA expression of PPARgamma, which was down-regulated by Borrelidin, was not changed by KLF3 and KLF7 siRNA treatment. In contrast, expression of PPARgamma in GATA-3 siRNA-treated cells was not significantly different from that of control siRNA-treated cells. Borrelidin significantly inhibited lipid accumulation in control siRNA-treated cells, and treatment with GATA-3 siRNA slightly reduced the inhibitory effect of Borrelidin. These results indicate that Borrelidin inhibited adipocyte differentiation partially via GATA-3.
Truncated borrelidin analogues: synthesis by sequential cross metathesis/olefination for the southern fragment and biological evaluation.[Pubmed:27523181]
Org Biomol Chem. 2016 Sep 21;14(35):8261-9.
The construction of novel Borrelidin analogues is reported in which the northern fragment is truncated to a simple hydroxyundecanecarboxylate and the original cyclopentanecarboxylic acid in the southern fragment is replaced with different six-membered rings. The required precursors were prepared by cross metathesis of the appropriate carbocycle-based homoallylic alcohol with crotonaldehyde followed by HWE olefination of the resulting enal with bromocyanophosphonate. The key aldehyde for intramolecular cross coupling was accessible by oxidation of the hydroxy group of the linked undecanecarboxylate unit. Grignard mediated macrocyclization finally yielded the Borrelidin related products. The investigation is complemented by SAR studies and quantum-chemical calculations.


