ArtemetherSemi-synthetic derivative of artemisinin CAS# 71963-77-4 |
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
- SB743921
Catalog No.:BCC4559
CAS No.:940929-33-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 71963-77-4 | SDF | Download SDF |
| PubChem ID | 68911 | Appearance | White powder |
| Formula | C16H26O5 | M.Wt | 298.38 |
| Type of Compound | Sesquiterpenes | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (335.15 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| SMILES | CC1CCC2C(C(OC3C24C1CCC(O3)(OO4)C)OC)C | ||
| Standard InChIKey | SXYIRMFQILZOAM-HVNFFKDJSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Artemether is a novel sonosensitizer,which has neurotoxicity, anti-schistosomiasis, anticancer and antitumor activities. It is an antimalarial for the treatment of resistant strains of falciparum malaria. |
| Targets | Antifection |
| In vivo | Potential sonodynamic anticancer activities of artemether and liposome-encapsulated artemether.[Pubmed: 25691357]Chem Commun (Camb). 2015 Mar 3;51(22):4681-4.The potential application of Artemether as a novel sonosensitizer for sonodynamic therapy (SDT) was explored and illustrated for the first time. In addition, liposome-encapsulated Artemether exhibited significantly enhanced sonodynamic anticancer activity. Our findings indicated that artemisinin derivatives may serve as a new kind of sonosensitizer for SDT. Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia.[Pubmed: 12020890]Acta Trop. 2002 May;82(2):175-81.Two decades ago, a group of Chinese scientists discovered the antischistosomal properties of Artemether, a derivative of the antimalarial drug artemisinin. However, it was only recently that the importance of this finding was recognized internationally, following a collaborative effort between Chinese, European and African scientists, who investigated the effects of Artemether against the major human schistosome species.
|
| Animal Research | Fatal neurotoxicity of arteether and artemether.[Pubmed: 7943542]Enhanced antimalarial activity by a novel artemether-lumefantrine lipid emulsion for parenteral administration.[Pubmed: 24982079]Antimicrob Agents Chemother. 2014 Oct;58(10):5658-65.Artemether and lumefantrine (also known as benflumetol) are difficult to formulate for parenteral administration because of their low aqueous solubility. Cremophor EL as an emulsion excipient has been shown to cause serious side effects.
Am J Trop Med Hyg. 1994 Sep;51(3):251-9.Artemisinin (qinghaosu) and several derivatives have been developed and are in use as antimalarial drugs but scant information is available regarding animal or human toxicity. Following a eight-day, multiple-dose, pharmacokinetic study of arteether (AE) (10 mg/kg/day [n = 6] and 20 mg/kg/day [n = 6]) in dogs, all high-dose animals displayed a progressive syndrome of clinical neurologic defects with progressive cardiorespiratory collapse and death in five of six animals. Neurologic findings included gait disturbances, loss of spinal and pain response reflexes, and prominent loss of brain stem and eye reflexes. Animals had prolongation of QT interval corrected for rate (QTc) on electrocardiograms (ECGs) with bizarre ST-T segment changes. Prominent neuropathic lesions were noted to be primarily limited to the pons and medulla. Similar lesions with dose-related severity were noted in eight other dogs studied in a second study with intramuscular (IM) administration of AE in sesame oil during a 28-day, dose-ranging study using 5, 10, 15, and 20 mg/kg/day. Injury, graded by a pathologist blinded to the dose group, showed a dose-related, region-specific injury in all animals that was most pronounced in the pons. Further studies in Sprague-Dawley rats using IM administration of AE and Artemether (AM) at a dose of 12.5-50 mg/kg/day for 28 days confirmed the onset of a clinical neurologic syndrome with dose-related changes in body weight, activity, and seizure-like activity, stereotypic movement disorders, and ECG changes |

Artemether Dilution Calculator

Artemether Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3514 mL | 16.7572 mL | 33.5143 mL | 67.0286 mL | 83.7858 mL |
| 5 mM | 0.6703 mL | 3.3514 mL | 6.7029 mL | 13.4057 mL | 16.7572 mL |
| 10 mM | 0.3351 mL | 1.6757 mL | 3.3514 mL | 6.7029 mL | 8.3786 mL |
| 50 mM | 0.067 mL | 0.3351 mL | 0.6703 mL | 1.3406 mL | 1.6757 mL |
| 100 mM | 0.0335 mL | 0.1676 mL | 0.3351 mL | 0.6703 mL | 0.8379 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
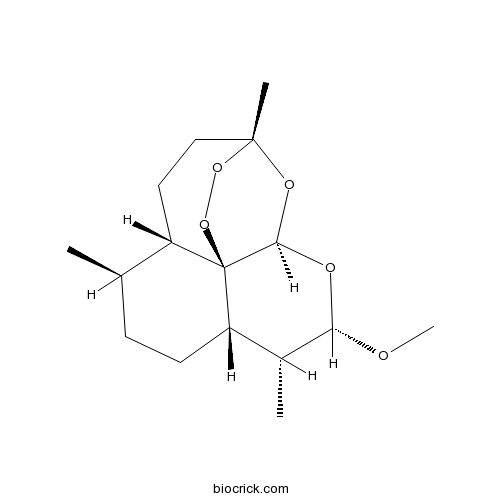
Artemether is a semi-synthetic derivative of artemisinin (qinghaosu), a naturally occurring peroxide lactone derived from a traditional Chinese herbal remedy Artemisia annua L., to enhance the solubility and antimalarial activity of its parental compound. Artemether exhibits potent antimalarial and antischistosomal activities against malarial parasites and Schistosoma japonicum infections. Although it has not been well elucidated, an endoperoxide bridge within the chemical structure of Artemether might be the key characteristic considered to be responsible for its antimalarial and antischistosomal activities, which can be metabolized by malarial or schistosomal parasites, under in vivo conditions, generating detrimental oxygen radicals to attack parasite macromolecules.
Reference
Xiao Shuhua and Brian A. Catto. In vitro and in vivo studies of the effect of artemether on Schistosoma mansoni. ANTIMICROBIAL AGENTS AND CHEMOTHERAPY 1989, 1557-1562
- Leiocarposide
Catalog No.:BCC8196
CAS No.:71953-77-0
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
- Sarmentosin
Catalog No.:BCN4275
CAS No.:71933-54-5
- ProINDY
Catalog No.:BCC6350
CAS No.:719277-30-2
- S-Adenosyl-L-methionine tosylate
Catalog No.:BCN2230
CAS No.:71914-80-2
- 2-Amino-5-chlorobenzophenone
Catalog No.:BCC8535
CAS No.:719-59-5
- PHA-793887
Catalog No.:BCC2521
CAS No.:718630-59-2
- Camaldulenic acid
Catalog No.:BCN3928
CAS No.:71850-15-2
- Borrelidin
Catalog No.:BCC7964
CAS No.:7184-60-3
- Sativan
Catalog No.:BCN6815
CAS No.:71831-00-0
- Ivermectin B1a
Catalog No.:BCC9005
CAS No.:71827-03-7
- Demethoxyencecalinol
Catalog No.:BCN7765
CAS No.:71822-00-9
- Fmoc-Asp(OtBu)-OH
Catalog No.:BCC3469
CAS No.:71989-14-5
- Fmoc-Asn-OH
Catalog No.:BCC3079
CAS No.:71989-16-7
- Fmoc-Glu(OtBu)-OH
Catalog No.:BCC3494
CAS No.:71989-18-9
- Fmoc-Gln-OH
Catalog No.:BCC3483
CAS No.:71989-20-3
- Fmoc-Ile-OH
Catalog No.:BCC3505
CAS No.:71989-23-6
- Fmoc-Lys(Boc)-OH
Catalog No.:BCC3516
CAS No.:71989-26-9
- Fmoc-Met-OH
Catalog No.:BCC3528
CAS No.:71989-28-1
- Fmoc-Pro-OH
Catalog No.:BCC3538
CAS No.:71989-31-6
- Fmoc-Ser(tBu)-OH
Catalog No.:BCC3544
CAS No.:71989-33-8
- Fmoc-Thr(tBu)-OH
Catalog No.:BCC3552
CAS No.:71989-35-0
- Fmoc-Tyr(tBu)-OH
Catalog No.:BCC3567
CAS No.:71989-38-3
- Fmoc-Tyr(Bzl)-OH
Catalog No.:BCC3564
CAS No.:71989-40-7
Potential sonodynamic anticancer activities of artemether and liposome-encapsulated artemether.[Pubmed:25691357]
Chem Commun (Camb). 2015 Mar 18;51(22):4681-4.
The potential application of Artemether as a novel sonosensitizer for sonodynamic therapy (SDT) was explored and illustrated for the first time. In addition, liposome-encapsulated Artemether exhibited significantly enhanced sonodynamic anticancer activity. Our findings indicated that artemisinin derivatives may serve as a new kind of sonosensitizer for SDT.
Fatal neurotoxicity of arteether and artemether.[Pubmed:7943542]
Am J Trop Med Hyg. 1994 Sep;51(3):251-9.
Artemisinin (qinghaosu) and several derivatives have been developed and are in use as antimalarial drugs but scant information is available regarding animal or human toxicity. Following a eight-day, multiple-dose, pharmacokinetic study of arteether (AE) (10 mg/kg/day [n = 6] and 20 mg/kg/day [n = 6]) in dogs, all high-dose animals displayed a progressive syndrome of clinical neurologic defects with progressive cardiorespiratory collapse and death in five of six animals. Neurologic findings included gait disturbances, loss of spinal and pain response reflexes, and prominent loss of brain stem and eye reflexes. Animals had prolongation of QT interval corrected for rate (QTc) on electrocardiograms (ECGs) with bizarre ST-T segment changes. Prominent neuropathic lesions were noted to be primarily limited to the pons and medulla. Similar lesions with dose-related severity were noted in eight other dogs studied in a second study with intramuscular (IM) administration of AE in sesame oil during a 28-day, dose-ranging study using 5, 10, 15, and 20 mg/kg/day. Injury, graded by a pathologist blinded to the dose group, showed a dose-related, region-specific injury in all animals that was most pronounced in the pons. Further studies in Sprague-Dawley rats using IM administration of AE and Artemether (AM) at a dose of 12.5-50 mg/kg/day for 28 days confirmed the onset of a clinical neurologic syndrome with dose-related changes in body weight, activity, and seizure-like activity, stereotypic movement disorders, and ECG changes.(ABSTRACT TRUNCATED AT 250 WORDS)
Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia.[Pubmed:12020890]
Acta Trop. 2002 May;82(2):175-81.
Two decades ago, a group of Chinese scientists discovered the antischistosomal properties of Artemether, a derivative of the antimalarial drug artemisinin. However, it was only recently that the importance of this finding was recognized internationally, following a collaborative effort between Chinese, European and African scientists, who investigated the effects of Artemether against the major human schistosome species. Laboratory studies revealed that Artemether exhibits the highest activity against juvenile stages of the parasites, while adult worms are significantly less susceptible. There was no indication of neurotoxicity following repeated high doses of Artemether given fortnightly for up to 5 months. Randomized controlled clinical trials confirmed that Artemether, orally administered at a dose of 6 mg/kg once every 2-3 weeks, results in no drug-related adverse effects, and significantly reduces the incidence and intensity of schistosome infections. The risk that these treatment regimens might select for resistance, particularly for resistant-plasmodia, appears to be low. Combined treatment with Artemether and praziquantel, given to animals harbouring juvenile and adult schistosome worms, resulted in significantly higher worm burden reductions than each drug administered singly. In conclusion, Artemether-integrated with other control strategies-has considerable potential for reducing the current burden of schistosomiasis in different epidemiological settings.
Enhanced antimalarial activity by a novel artemether-lumefantrine lipid emulsion for parenteral administration.[Pubmed:24982079]
Antimicrob Agents Chemother. 2014 Oct;58(10):5658-65.
Artemether and lumefantrine (also known as benflumetol) are difficult to formulate for parenteral administration because of their low aqueous solubility. Cremophor EL as an emulsion excipient has been shown to cause serious side effects. This study reports a method of preparation and the therapeutic efficacies of novel lipid emulsion (LE) delivery systems with Artemether, lumefantrine, or Artemether in combination with lumefantrine, for parenteral administration. Their physical and chemical stabilities were also evaluated. Furthermore, the in vivo antimalarial activities of the lipid emulsions developed were tested in Plasmodium berghei-infected mice. Artemether, lumefantrine, or Artemether in combination with lumefantrine was encapsulated in an oil phase, and the in vivo performance was assessed by comparison with artesunate for injection. It was found that the lumefantrine lipid emulsion (LUM-LE) and Artemether-lumefantrine lipid emulsion (ARM-LUM-LE-3) (1:6) began to decrease the parasitemia levels after only 3 days, and the parasitemia inhibition was 90% at doses of 0.32 and 0.27 mg/kg, respectively, with immediate antimalarial effects greater than those of the positive-control group and constant antimalarial effects over 30 days. LUM-LE and ARM-LUM-LE-3 demonstrated the best performance in terms of chemical and physical stabilities and antiplasmodial efficacy, with a mean particle size of 150 nm, and they have many favorable properties for parenteral administration, such as biocompatibility, physical stability, and ease of preparation.


