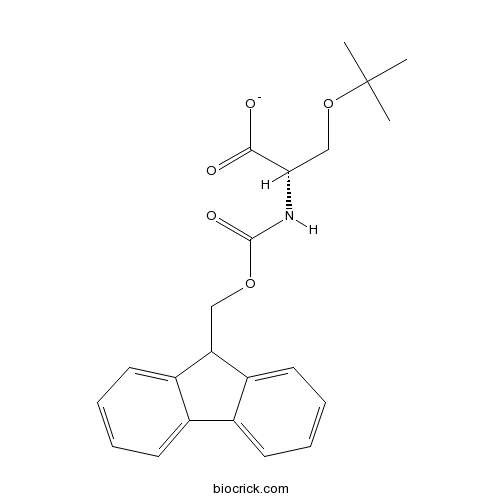
Fmoc-Ser(tBu)-OHCAS# 71989-33-8 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 71989-33-8 | SDF | Download SDF |
| PubChem ID | 7009542 | Appearance | Powder |
| Formula | C22H25NO5 | M.Wt | 383.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoate | ||
| SMILES | CC(C)(C)OCC(C(=O)[O-])NC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13 | ||
| Standard InChIKey | REITVGIIZHFVGU-IBGZPJMESA-M | ||
| Standard InChI | InChI=1S/C22H25NO5/c1-22(2,3)28-13-19(20(24)25)23-21(26)27-12-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,12-13H2,1-3H3,(H,23,26)(H,24,25)/p-1/t19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Ser(tBu)-OH Dilution Calculator

Fmoc-Ser(tBu)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6082 mL | 13.0412 mL | 26.0824 mL | 52.1648 mL | 65.2061 mL |
| 5 mM | 0.5216 mL | 2.6082 mL | 5.2165 mL | 10.433 mL | 13.0412 mL |
| 10 mM | 0.2608 mL | 1.3041 mL | 2.6082 mL | 5.2165 mL | 6.5206 mL |
| 50 mM | 0.0522 mL | 0.2608 mL | 0.5216 mL | 1.0433 mL | 1.3041 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2608 mL | 0.5216 mL | 0.6521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Ser(tBu)-OH
- Fmoc-Pro-OH
Catalog No.:BCC3538
CAS No.:71989-31-6
- Fmoc-Met-OH
Catalog No.:BCC3528
CAS No.:71989-28-1
- Fmoc-Lys(Boc)-OH
Catalog No.:BCC3516
CAS No.:71989-26-9
- Fmoc-Ile-OH
Catalog No.:BCC3505
CAS No.:71989-23-6
- Fmoc-Gln-OH
Catalog No.:BCC3483
CAS No.:71989-20-3
- Fmoc-Glu(OtBu)-OH
Catalog No.:BCC3494
CAS No.:71989-18-9
- Fmoc-Asn-OH
Catalog No.:BCC3079
CAS No.:71989-16-7
- Fmoc-Asp(OtBu)-OH
Catalog No.:BCC3469
CAS No.:71989-14-5
- Artemether
Catalog No.:BCN5973
CAS No.:71963-77-4
- Leiocarposide
Catalog No.:BCC8196
CAS No.:71953-77-0
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
- Sarmentosin
Catalog No.:BCN4275
CAS No.:71933-54-5
- Fmoc-Thr(tBu)-OH
Catalog No.:BCC3552
CAS No.:71989-35-0
- Fmoc-Tyr(tBu)-OH
Catalog No.:BCC3567
CAS No.:71989-38-3
- Fmoc-Tyr(Bzl)-OH
Catalog No.:BCC3564
CAS No.:71989-40-7
- H-Thr(tBu)-OMe.HCl
Catalog No.:BCC3107
CAS No.:71989-43-0
- Sulfathiazole
Catalog No.:BCC4859
CAS No.:72-14-0
- H-Val-OH
Catalog No.:BCC3138
CAS No.:72-18-4
- H-Thr-OH
Catalog No.:BCC3102
CAS No.:72-19-5
- Alizarin
Catalog No.:BCN3479
CAS No.:72-48-0
- 2,2-Bis(4-chlorophenyl)-1,1-dichloroethane
Catalog No.:BCC8492
CAS No.:72-54-8
- 2,2-Bis(4-chlorophenyl)-1,1-dichloroethylene
Catalog No.:BCC8493
CAS No.:72-55-9
- Metandienone
Catalog No.:BCC9025
CAS No.:72-63-9
- Chlorquinaldol
Catalog No.:BCC4648
CAS No.:72-80-0
Polymer-Supported Stereoselective Synthesis of Benzoxazino[4,3-b][1,2,5]thiadiazepinone 6,6-dioxides.[Pubmed:28825802]
ACS Comb Sci. 2017 Oct 9;19(10):670-674.
Herein, we report the stereoselective synthesis of trisubstituted benzoxazino[4,3-b][1,2,5]thiadiazepinone 6,6-dioxides from polymer-supported Fmoc-Ser(tBu)-OH and Fmoc-Thr(tBu)-OH. After the solid-phase synthesis of N-alkylated-N-sulfonylated intermediates using various 2-nitrobenzenesulfonyl chlorides and bromoketones, the target compounds were obtained via trifluoroacetic acid (TFA)-mediated cleavage from the resin, followed by cyclization of the diazepinone scaffold. Except for the threonine-based intermediates, the inclusion of triethylsilane (TES) in the cleavage cocktail yielded a specific configuration of the newly formed C(3) chiral center. The final cyclization resulted in minor or no inversion of the C(12a) stereocenter configuration.
Stereoselective Polymer-Supported Synthesis of Morpholine- and Thiomorpholine-3-carboxylic Acid Derivatives.[Pubmed:28085245]
ACS Comb Sci. 2017 Mar 13;19(3):173-180.
Herein we report the polymer-supported synthesis of 3,4-dihydro-2H-1,4-oxazine-3-carboxylic acid derivatives using immobilized Fmoc-Ser(tBu)-OH and Fmoc-Thr(tBu)-OH as the starting materials. After the solid-phase-synthesis of N-alkyl-N-sulfonyl/acyl intermediates, the target dihydrooxazines were obtained using trifluoroacetic acid-mediated cleavage from the resin. This approach was also studied for the preparation of dihydrothiazines from immobilized Fmoc-Cys(Trt)-OH. Inclusion of triethylsilane in the cleavage cocktail resulted in the stereoselective formation of the corresponding morpholine/thiomorpholine-3-carboxylic acids. Stereochemical studies revealed the specific configuration of the newly formed stereocenter and also the formation of stable N-acylmorpholine rotamers.


