Danoprevir (RG7227)HCV NS3/4A protease inhibitor CAS# 850876-88-9 |
- Daclatasvir (BMS-790052)
Catalog No.:BCC2533
CAS No.:1214735-16-6
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- MK-5172
Catalog No.:BCC1762
CAS No.:1350514-68-9
- MK-5172 sodium salt
Catalog No.:BCC1765
CAS No.:1425038-27-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Simeprevir
Catalog No.:BCC1949
CAS No.:923604-59-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 850876-88-9 | SDF | Download SDF |
| PubChem ID | 11285588 | Appearance | Powder |
| Formula | C35H46FN5O9S | M.Wt | 731.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ITMN-191; R7227; RO5190591; RG7227 | ||
| Solubility | DMSO : ≥ 100 mg/mL (136.64 mM) *"≥" means soluble, but saturation unknown. | ||
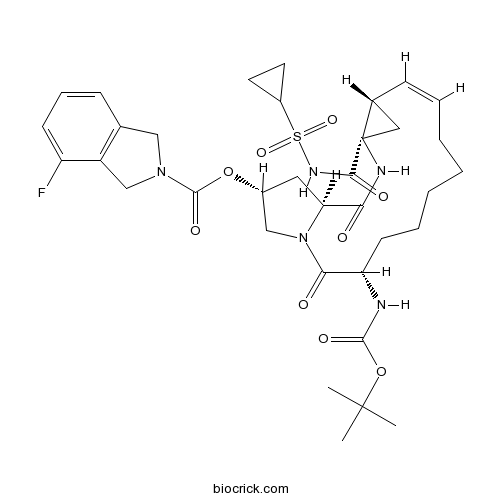
| Chemical Name | [(1S,4R,6S,7Z,14S,18R)-4-(cyclopropylsulfonylcarbamoyl)-14-[(2-methylpropan-2-yl)oxycarbonylamino]-2,15-dioxo-3,16-diazatricyclo[14.3.0.04,6]nonadec-7-en-18-yl] 4-fluoro-1,3-dihydroisoindole-2-carboxylate | ||
| SMILES | CC(C)(C)OC(=O)NC1CCCCCC=CC2CC2(NC(=O)C3CC(CN3C1=O)OC(=O)N4CC5=C(C4)C(=CC=C5)F)C(=O)NS(=O)(=O)C6CC6 | ||
| Standard InChIKey | ZVTDLPBHTSMEJZ-JSZLBQEHSA-N | ||
| Standard InChI | InChI=1S/C35H46FN5O9S/c1-34(2,3)50-32(45)37-27-13-8-6-4-5-7-11-22-17-35(22,31(44)39-51(47,48)24-14-15-24)38-29(42)28-16-23(19-41(28)30(27)43)49-33(46)40-18-21-10-9-12-26(36)25(21)20-40/h7,9-12,22-24,27-28H,4-6,8,13-20H2,1-3H3,(H,37,45)(H,38,42)(H,39,44)/b11-7-/t22-,23-,27+,28+,35-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Danoprevir is a highly selective and potent inhibitor of HCV NS3/4A protease with IC50 value of 0.2-3.5 nM. | |||||
| Targets | HCV NS3/4A protease | |||||
| IC50 | 0.2-3.5 nM | |||||

Danoprevir (RG7227) Dilution Calculator

Danoprevir (RG7227) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3664 mL | 6.8322 mL | 13.6644 mL | 27.3288 mL | 34.1609 mL |
| 5 mM | 0.2733 mL | 1.3664 mL | 2.7329 mL | 5.4658 mL | 6.8322 mL |
| 10 mM | 0.1366 mL | 0.6832 mL | 1.3664 mL | 2.7329 mL | 3.4161 mL |
| 50 mM | 0.0273 mL | 0.1366 mL | 0.2733 mL | 0.5466 mL | 0.6832 mL |
| 100 mM | 0.0137 mL | 0.0683 mL | 0.1366 mL | 0.2733 mL | 0.3416 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Danoprevir (R7227) is a potent and selective inhibitor of Hepatitis C Virus (HCV) NS3/4A protease, a chymotrypsin-like serine protease playing an essential role in the viral replication process of HCV, that non-covalently binds to and hence inhibits HCV NS3 protease with 50% inhibition concentration IC50 values ranging from 0.2 to 3.5 nM. X-ray crystallographic analysis has revealed that the cyclopropyl acylsulfonamide of danoprevir occupies the SI/SI’ pocket of HCV NS3 protease with the acyl carbonyl oxygen forming hydrogen bonds to Gly137 and Ser138 in the oxyanion hole of the protease active site and the acyl sulfonamide nitrogen forming a hydrogen bond with His57.
Reference
Jiang Y, Andrews SW, Condroski KR, Buckman B, Serebryany V, Wenglowsky S, Kennedy AL, Madduru MR, Wang B, Lyon M, Doherty GA, Woodard BT, Lemieux C, Do MG, Zhang H, Ballard J, Vigers G, Brandhuber BJ, Stengel P, Josey JA, Beigelman L, Blatt L, Seiwert SD. Discovery of Danoprevir (ITMN-191/R7227), a Highly Selective and Potent Inhibitor of Hepatitis C Virus (HCV) NS3/4A Protease. J Med Chem. 2013 May 28. [Epub ahead of print]
- Cinchonain IIa
Catalog No.:BCN7716
CAS No.:85081-23-8
- NBI-98782
Catalog No.:BCC4277
CAS No.:85081-18-1
- BX517(PDK1 inhibitor2)
Catalog No.:BCC6391
CAS No.:850717-64-5
- GSK269962A
Catalog No.:BCC5178
CAS No.:850664-21-0
- Alogliptin Benzoate
Catalog No.:BCC1341
CAS No.:850649-62-6
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
- Apigenin 5-O-neohesperidoside
Catalog No.:BCN6840
CAS No.:850630-40-9
- Norviburtinal
Catalog No.:BCN4399
CAS No.:85051-41-8
- Fmoc-Arg(Tos)-ol
Catalog No.:BCC2588
CAS No.:850330-29-9
- Rosin
Catalog No.:BCN5969
CAS No.:85026-55-7
- Cinchonain IIb
Catalog No.:BCN7738
CAS No.:85022-68-0
- Shikonofuran A
Catalog No.:BCN2826
CAS No.:85022-66-8
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- THIP hydrochloride
Catalog No.:BCC6803
CAS No.:85118-33-8
- 2-Methoxystypandrone
Catalog No.:BCN4400
CAS No.:85122-21-0
- PF9 tetrasodium salt
Catalog No.:BCC7854
CAS No.:851265-78-6
- Phospho-Glycogen Synthase Peptide-2 (substrate)
Catalog No.:BCC5747
CAS No.:851366-97-7
- (S)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6600
CAS No.:85148-82-9
- Curculigoside C
Catalog No.:BCN3696
CAS No.:851713-74-1
- OC000459
Catalog No.:BCC4507
CAS No.:851723-84-7, 950688-14-9 (sodium salt)
- PF 514273
Catalog No.:BCC7746
CAS No.:851728-60-4
- 3-(3-Chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepin-2-one
Catalog No.:BCC8587
CAS No.:85175-59-3
- ADX-47273
Catalog No.:BCC4598
CAS No.:851881-60-2
- RuBi-4AP
Catalog No.:BCC6044
CAS No.:851956-02-0
Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN-191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study.[Pubmed:21145848]
J Hepatol. 2011 Jun;54(6):1130-6.
BACKGROUND & AIMS: Danoprevir is a potent and selective inhibitor of the hepatitis C virus (HCV) NS3/4A serine protease. The present study assessed the safety, pharmacokinetics, and antiviral activity of danoprevir in a randomized, placebo-controlled, 14-day multiple ascending dose study in patients with chronic HCV genotype 1 infection. METHODS: Four cohorts of treatment-naive (TN) patients (100 mg q12 h, 100 mg q8 h, 200 mg q12 h, 200 mg q8 h) and one cohort of non-responders (NR) to prior pegylated interferon alfa-ribavirin treatment (300 mg q12 h) were investigated. RESULTS: Danoprevir was safe and well tolerated; adverse events were generally mild, transient and were not associated with treatment group or dose level. Danoprevir displayed a slightly more than proportional increase in exposure with increasing daily dose and was rapidly eliminated from the plasma compartment. Maximal decreases in HCV RNA were: -3.9 log(10)IU/ml and -3.2 log(10)IU/ml in TN receiving 200 mg q8 h and 200 mg q12 h, respectively. End of treatment viral decline in these two cohorts was within 0.1 log(10)IU/ml of the viral load nadir. HCV RNA reduction in NR was more modest than that observed in upper dose TN cohorts. The overall incidence of viral rebound was low (10/37) and was associated with the R155K substitution in NS3 regardless of the HCV subtype. CONCLUSIONS: Danoprevir was safe and well tolerated when administered for 14 days in patients with chronic HCV genotype 1 infection. Treatment resulted in sustained, multi-log(10) IU/ml reductions in HCV RNA in upper dose cohorts. These results support further clinical evaluation of danoprevir in patients with chronic HCV.
Antiviral activity of danoprevir (ITMN-191/RG7227) in combination with pegylated interferon alpha-2a and ribavirin in patients with hepatitis C.[Pubmed:21791662]
J Infect Dis. 2011 Aug 15;204(4):601-8.
BACKGROUND: Current therapy options for patients with chronic hepatitis C virus (HCV) infection genotype 1 are effective in <50%. Danoprevir (ITMN-191/RG7227) is a potent, selective, and orally active inhibitor of the HCV NS3/4A serine protease. METHODS: The safety and antiviral efficacy of danoprevir was examined over 14 days in combination with pegylated interferon alpha-2a (180 mug once weekly) and ribavirin (1000-1200 mg/day) in a double-blind, placebo-controlled, phase 1b, multiple ascending dose study consisting of 6 dose cohorts (400 mg, 600 mg, and 900 mg twice daily and 100 mg, 200 mg, and 300 mg 3 times daily). RESULTS: Danoprevir in combination with pegylated interferon alpha-2a and ribavirin was safe and generally well tolerated. The median change in HCV RNA level from baseline to the end of treatment with danoprevir at 400 mg, 600 mg, and 900 mg twice daily was -4.7 log(10) IU/mL, -5.4 log(10) IU/mL, and -5.3 log(10) IU/mL, respectively, and at 100 mg, 200 mg, and 300 mg 3 times daily was -5.5 log(10) IU/mL, -5.7 log(10) IU/mL, and -5.6 log(10) IU/mL, respectively. Placebo administered in combination with standard of care resulted in median decrease in HCV RNA level of -2.6 log(10) IU/mL (with twice daily regimen) and -2.0 log(10) IU/mL (with 3 times daily regimen). CONCLUSIONS: Our study showed substantial antiviral efficacy of danoprevir in combination with pegylated interferon alpha-2a and ribavirin. Exploration of the safety and antiviral efficacy of danoprevir in longer clinical studies is warranted.
Virologic escape during danoprevir (ITMN-191/RG7227) monotherapy is hepatitis C virus subtype dependent and associated with R155K substitution.[Pubmed:22064535]
Antimicrob Agents Chemother. 2012 Jan;56(1):271-9.
Danoprevir is a hepatitis C virus (HCV) NS3/4A protease inhibitor that promotes multi-log(10) reductions in HCV RNA when administered as a 14-day monotherapy to patients with genotype 1 chronic HCV. Of these patients, 14/37 experienced a continuous decline in HCV RNA, 13/37 a plateau, and 10/37 a rebound. The rebound and continuous-decline groups experienced similar median declines in HCV RNA through day 7, but their results diverged notably at day 14. Plateau group patients experienced a lesser, but sustained, median HCV RNA decline. Baseline danoprevir susceptibility was similar across response groups but was reduced significantly at day 14 in the rebound group. Viral rebound in genotype 1b was uncommon (found in 2/23 patients). Population-based sequence analysis of NS3 and NS4A identified treatment-emergent substitutions at four amino acid positions in the protease domain of NS3 (positions 71, 155, 168, and 170), but only two (155 and 168) were in close proximity to the danoprevir binding site and carried substitutions that impacted danoprevir potency. R155K was the predominant route to reduced danoprevir susceptibility and was observed in virus isolated from all 10 rebound, 2/13 plateau, and 1/14 continuous-decline patients. Virus in one rebound patient additionally carried partial R155Q and D168E substitutions. Treatment-emergent substitutions in plateau patients were less frequently observed and more variable. Single-rebound patients carried virus with R155Q, D168V, or D168T. Clonal sequence analysis and drug susceptibility testing indicated that only a single patient displayed multiple resistance pathways. These data indicate the ascendant importance of R155K for viral escape during danoprevir treatment and may have implications for the clinical use of this agent.


