THIP hydrochlorideGABAA agonist CAS# 85118-33-8 |
- Lomeguatrib
Catalog No.:BCC1133
CAS No.:192441-08-0
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85118-33-8 | SDF | Download SDF |
| PubChem ID | 5702253 | Appearance | Powder |
| Formula | C6H9ClN2O2 | M.Wt | 176.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Gaboxadol | ||
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
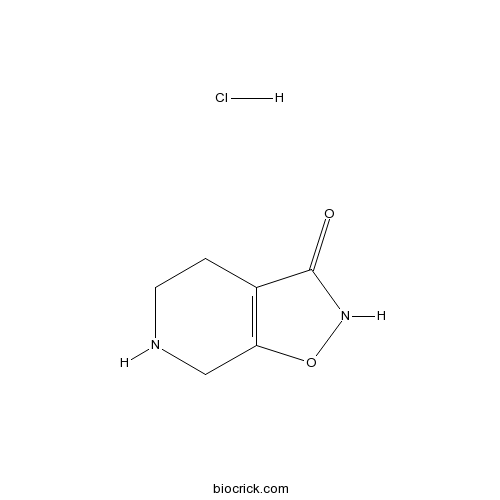
| Chemical Name | 4,5,6,7-tetrahydro-[1,2]oxazolo[5,4-c]pyridin-3-one;hydrochloride | ||
| SMILES | C1CNCC2=C1C(=O)NO2.Cl | ||
| Standard InChIKey | ZDZDSZQYRBZPNN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H8N2O2.ClH/c9-6-4-1-2-7-3-5(4)10-8-6;/h7H,1-3H2,(H,8,9);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Systemically active GABAA receptor agonist and GABAA-ρ receptor antagonist. Displays antinociceptive, anticonvulsant and sedative effects. Hypnotic agent that enhances delta activity within non-REM sleep in rats. |

THIP hydrochloride Dilution Calculator

THIP hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6625 mL | 28.3126 mL | 56.6251 mL | 113.2503 mL | 141.5629 mL |
| 5 mM | 1.1325 mL | 5.6625 mL | 11.325 mL | 22.6501 mL | 28.3126 mL |
| 10 mM | 0.5663 mL | 2.8313 mL | 5.6625 mL | 11.325 mL | 14.1563 mL |
| 50 mM | 0.1133 mL | 0.5663 mL | 1.1325 mL | 2.265 mL | 2.8313 mL |
| 100 mM | 0.0566 mL | 0.2831 mL | 0.5663 mL | 1.1325 mL | 1.4156 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Cinchonain IIa
Catalog No.:BCN7716
CAS No.:85081-23-8
- NBI-98782
Catalog No.:BCC4277
CAS No.:85081-18-1
- BX517(PDK1 inhibitor2)
Catalog No.:BCC6391
CAS No.:850717-64-5
- GSK269962A
Catalog No.:BCC5178
CAS No.:850664-21-0
- Alogliptin Benzoate
Catalog No.:BCC1341
CAS No.:850649-62-6
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
- Apigenin 5-O-neohesperidoside
Catalog No.:BCN6840
CAS No.:850630-40-9
- Norviburtinal
Catalog No.:BCN4399
CAS No.:85051-41-8
- Fmoc-Arg(Tos)-ol
Catalog No.:BCC2588
CAS No.:850330-29-9
- Rosin
Catalog No.:BCN5969
CAS No.:85026-55-7
- 2-Methoxystypandrone
Catalog No.:BCN4400
CAS No.:85122-21-0
- PF9 tetrasodium salt
Catalog No.:BCC7854
CAS No.:851265-78-6
- Phospho-Glycogen Synthase Peptide-2 (substrate)
Catalog No.:BCC5747
CAS No.:851366-97-7
- (S)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6600
CAS No.:85148-82-9
- Curculigoside C
Catalog No.:BCN3696
CAS No.:851713-74-1
- OC000459
Catalog No.:BCC4507
CAS No.:851723-84-7, 950688-14-9 (sodium salt)
- PF 514273
Catalog No.:BCC7746
CAS No.:851728-60-4
- 3-(3-Chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepin-2-one
Catalog No.:BCC8587
CAS No.:85175-59-3
- ADX-47273
Catalog No.:BCC4598
CAS No.:851881-60-2
- RuBi-4AP
Catalog No.:BCC6044
CAS No.:851956-02-0
- TOK-001
Catalog No.:BCC3910
CAS No.:851983-85-2
- BAPTA
Catalog No.:BCC7483
CAS No.:85233-19-8
Enantiotropically related polymorphs of gaboxadol hydrochloride.[Pubmed:24192165]
Acta Crystallogr C. 2013 Nov;69(Pt 11):1234-7.
Gaboxadol hydrochloride, also known as THIP hydrochloride (systematic name: 3-hydroxy-4,5,6,7-tetrahydro-1,2-oxazolo[5,4-c]pyridin-6-ium chloride), C6H9N2O2(+).Cl(-), exists as two enantiotropically related polymorphs. Transformation between the polymorphs occurs in a single-crystal-to-single-crystal manner at 221 K, and the enthalpy of transformation from the high-temperature form to the low-temperature form is -0.7 kJ mol(-1). Single-crystal structures have been determined at 298 and 220 K. At 298 K, the structure is triclinic (space group P overline 1), with two formula units in the crystallographic asymmetric unit. At 220 K, the structure is monoclinic (space group I2/a), with one formula unit in the asymmetric unit. The structures contain identical hydrogen-bonded layers and the transformation between the polymorphs corresponds to a shift of adjacent layers relative to each other. The transformation is shown to be reversible by differential scanning calorimetry and variable-temperature powder X-ray diffraction.
The role of GABAergic system on the inhibitory effect of ghrelin on food intake in neonatal chicks.[Pubmed:22613635]
Neurosci Lett. 2012 Jun 27;520(1):82-6.
Ghrelin is a gut-brain peptide that has a stimulatory effect on food intake in mammals. In contrast, this peptide decreases food intake in neonatal chicks when injected intracerebroventricularly (ICV). In mammals, neuropeptide Y (NPY) mediates the orexigenic effect of ghrelin whereas in chicks it appears that corticotrophin releasing factor (CRF) is partially involved in the inhibitory effect of ghrelin on food intake. Gamma aminobutyric acid (GABA) has a stimulatory effect on food intake in mammals and birds. In this study we investigated whether the anorectic effect of ghrelin is mediated by the GABAergic system. In Experiment 1, 3h-fasted chicks were given an ICV injection of chicken ghrelin and picrotoxin, a GABA(A) receptors antagonist. Picrotoxin decreased food intake compared to the control chicks indicating a stimulatory effect of GABA(A) receptors on food intake. However, picrotoxin did not alter the inhibitory effect of ghrelin on food intake. In Experiment 2, THIP hydrochloride, a GABA(A) receptor agonist, was used in place of picrotoxin. THIP hydrochloride appeared to partially attenuate the decrease in food intake induced by ghrelin at 30 min postinjection. In Experiment 3, the effect of ICV injection of chicken ghrelin on gene expression of glutamate decarboxylase (GAD)(1) and GAD(2), GABA synthesis enzymes in the brain stem including hypothalamus, was investigated. The ICV injection of chicken ghrelin significantly reduced GAD(2) gene expression. These findings suggest that ghrelin may decrease food intake in neonatal chicks by reducing GABA synthesis and thereby GABA release within brain feeding centers.
Gaboxadol. Lundbeck/Merck.[Pubmed:15298075]
Curr Opin Investig Drugs. 2004 Jul;5(7):766-73.
H Lundbeck A/S, in collaboration with Merck & Co Inc, is developing gaboxadol, a GABAA agonist, for the potential treatment of sleep disorders. The compound is currently undergoing phase III clinical trials.
GABAc receptors: relatively simple transmitter -gated ion channels?[Pubmed:8885697]
Trends Pharmacol Sci. 1996 Sep;17(9):319-23.
The inhibitory neurotransmitter, GABA, activates a variety of receptors in all areas of the CNS. Two major subtypes of GABA receptors are well known: (1) GABAA receptors are ligand-gated Cl- channels that consist of a heteromeric mixture of protein subunits forming a pentameric structure, and (2) GABAB receptors couple to Ca2+ and K+ channels via G proteins and second messengers. Here, Graham Johnston discusses evidence for a third major subclass of GABA receptors. GABAC receptors appear to be relatively simple ligand-gated Cl- channels with a distinctive pharmacology, in that they are not blocked by bicuculline and not modulated by barbiturates, benzodiazepines or neuroactive steroids. Compared with GABAA receptors, GABAC receptors are activated at lower concentrations of GABA and are less liable to desensitization. In addition, their channels open for a longer time. The pharmacology of these novel subtypes of GABA receptors may yield important therapeutic agents.
4,5,6,7-Tetrahydroisothiazolo[5,4-c]pyridin-3-ol and related analogues of THIP. Synthesis and biological activity.[Pubmed:6304315]
J Med Chem. 1983 Jun;26(6):895-900.
The thio analogues of the GABA (gamma-aminobutyric acid) agonist THIP (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol), the GABA uptake inhibitor THPO (4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol), and the glycine antagonist THAZ (5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol) have been synthesized and tested biologically on single neurons in the cat spinal cord and in vitro by using synaptic membrane preparations obtained from rat brains. In contrast to THIP, thio-THIP (4,5,6,7-tetrahydroisothiazolo[5,4-c]pyridin-3-ol, 5) was only a weak GABA agonist. Thio-THPO (4,5,6,7-tetrahydroisothiazolo[4,5-c]pyridin-3-ol, 10) was slightly weaker than THPO as an inhibitor of GABA uptake in vitro, and these two compounds were approximately equipotent in enhancing the inhibition of the firing of cat spinal neurons by GABA. Like THAZ and structurally related bicyclic isoxazole zwitterions, thio-THAZ (5,6,7,8-tetrahydro-4H-isothiazolo[4,5-d]azepin-3-ol, 15) was an antagonist at glycine receptors on cat spinal neurons. The I/U ratios, which reflect the ability of neutral amino acids to penetrate the blood-brain barrier (BBB), were calculated for 5 (I/U = 16), 10 (63), and 15 (200). These low I/U ratios, compared with the findings that THIP (I/U = 500 or 1500) and THPO (I/U = 2500) enter the brain after systemic administration, suggest that the thio analogues may penetrate the BBB very easily.


