Curculigoside CCAS# 851713-74-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 851713-74-1 | SDF | Download SDF |
| PubChem ID | 102004677 | Appearance | Powder |
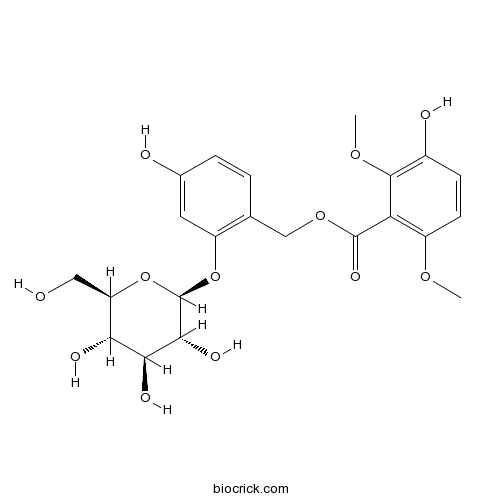
| Formula | C22H26O12 | M.Wt | 482.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [4-hydroxy-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]methyl 3-hydroxy-2,6-dimethoxybenzoate | ||
| SMILES | COC1=C(C(=C(C=C1)O)OC)C(=O)OCC2=C(C=C(C=C2)O)OC3C(C(C(C(O3)CO)O)O)O | ||
| Standard InChIKey | DSPUSYGYNSWPGB-DRASZATQSA-N | ||
| Standard InChI | InChI=1S/C22H26O12/c1-30-13-6-5-12(25)20(31-2)16(13)21(29)32-9-10-3-4-11(24)7-14(10)33-22-19(28)18(27)17(26)15(8-23)34-22/h3-7,15,17-19,22-28H,8-9H2,1-2H3/t15-,17-,18+,19-,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Acta Botanica Sinica.2004;46(5):621-624.Curculigoside C, a new phenolic glucoside from rhizomes of {\sl Curculigo orchioides}.[Reference: WebLink]
|

Curculigoside C Dilution Calculator

Curculigoside C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.073 mL | 10.3648 mL | 20.7297 mL | 41.4594 mL | 51.8242 mL |
| 5 mM | 0.4146 mL | 2.073 mL | 4.1459 mL | 8.2919 mL | 10.3648 mL |
| 10 mM | 0.2073 mL | 1.0365 mL | 2.073 mL | 4.1459 mL | 5.1824 mL |
| 50 mM | 0.0415 mL | 0.2073 mL | 0.4146 mL | 0.8292 mL | 1.0365 mL |
| 100 mM | 0.0207 mL | 0.1036 mL | 0.2073 mL | 0.4146 mL | 0.5182 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (S)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6600
CAS No.:85148-82-9
- Phospho-Glycogen Synthase Peptide-2 (substrate)
Catalog No.:BCC5747
CAS No.:851366-97-7
- PF9 tetrasodium salt
Catalog No.:BCC7854
CAS No.:851265-78-6
- 2-Methoxystypandrone
Catalog No.:BCN4400
CAS No.:85122-21-0
- THIP hydrochloride
Catalog No.:BCC6803
CAS No.:85118-33-8
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Cinchonain IIa
Catalog No.:BCN7716
CAS No.:85081-23-8
- NBI-98782
Catalog No.:BCC4277
CAS No.:85081-18-1
- BX517(PDK1 inhibitor2)
Catalog No.:BCC6391
CAS No.:850717-64-5
- GSK269962A
Catalog No.:BCC5178
CAS No.:850664-21-0
- Alogliptin Benzoate
Catalog No.:BCC1341
CAS No.:850649-62-6
- OC000459
Catalog No.:BCC4507
CAS No.:851723-84-7, 950688-14-9 (sodium salt)
- PF 514273
Catalog No.:BCC7746
CAS No.:851728-60-4
- 3-(3-Chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepin-2-one
Catalog No.:BCC8587
CAS No.:85175-59-3
- ADX-47273
Catalog No.:BCC4598
CAS No.:851881-60-2
- RuBi-4AP
Catalog No.:BCC6044
CAS No.:851956-02-0
- TOK-001
Catalog No.:BCC3910
CAS No.:851983-85-2
- BAPTA
Catalog No.:BCC7483
CAS No.:85233-19-8
- 6-Methyl-7-O-methylaromadendrin
Catalog No.:BCN4010
CAS No.:852385-13-8
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
- Dovitinib Dilactic acid
Catalog No.:BCC3771
CAS No.:852433-84-2
Pharmacokinetic and Metabolism Studies of Curculigoside C by UPLC-MS/MS and UPLC-QTOF-MS.[Pubmed:30577595]
Molecules. 2018 Dec 21;24(1). pii: molecules24010021.
Pharmacokinetic and metabolism studies were carried out on Curculigoside C (CC), a natural product with good antioxidant and neuroprotective effects, with the purpose of investigating the effects of the hydroxyl group at C-3' in curculigoside. A rapid and sensitive method with UPLC-MS was developed and fully validated for the first time in the pharmacokinetic analysis for quantification of CC in rat plasma. The assay was linear (R(2) > 0.9984) over the concentration range of 1(-)2500 ng/mL, with the lower limit of quantification (LLOQ) being 1 ng/mL. The intra-day and inter-day precision (expressed as relative standard deviation, RSD) ranged from 4.10% to 5.51% and 5.24% to 6.81%, respectively. The accuracy (relative error, RE) ranged from -3.28% to 0.56% and -5.83% to -1.44%, respectively. The recoveries ranged from 92.14% to 95.22%. This method was then applied to a pharmacokinetic study of rats after intragastric administration of 15, 30 and 60 mg/kg CC. The results revealed that CC exhibited rapid oral absorption (Tmax = 0.106 h, 0.111 h, and 0.111 h, respectively), high elimination (t1/2 = 2.022 h, 2.061 h, and 2.048 h, respectively) and low absolute bioavailability (2.01, 2.13, and 2.39%, respectively). Furthermore, an investigation on the metabolism of CC was performed by UPLC-QTOF-MS(E). Twelve metabolites of CC from plasma, bile, urine and faeces of rats were confirmed. The main metabolic pathways of CC, which involve dehydration, glucosylation, desaturation, formylation, cysteine conjugation, demethylation and sulfonation, were profiled. In conclusion, this research has developed a sensitive quantitative method and demonstrated the metabolism of CC in vivo.
Enhanced Synthesis of Curculigoside by Stress and Amino Acids in Static Culture of Curculigo orchioides Gaertn (Kali Musli).[Pubmed:27365988]
Pharmacognosy Res. 2016 Jul-Sep;8(3):193-8.
BACKGROUND: Curculigo orchioides Gaertn (Kali musli; Family: Hypoxidaceae) is an endangered medicinal plant used for many medicinal purposes such as impotency, aphrodisiac, tonic, jaundice, and skin ailments. Its hepatoprotective, antioxidant, and anti-cancerous potential have also been evaluated by many scientists. OBJECTIVE: The objective of this study is to enhance the Curculigoside Content in tissue culture of C. orchioides. MATERIALS AND METHODS: The present study deals with the enhancement of an active compound of C. orchioides by incorporating various concentration of phenylalanine (Phe), tyrosine, (20, 40, 60, and 80 mg/100 ml), chromium (Cr) and nickel (Ni) (1, 2, 3, 4, and 5 ppm) into Zenk media in controlled and aseptic conditions. RESULTS: Plant secondary metabolites are unique sources for pharmaceuticals, food additives, flavors, and industrially important biochemicals. Accumulation of such metabolites often occurs in plants subjected to stresses including various elicitors or signal molecules. A significantly remarkable enhancement in all induced samples was noted. Curculigoside Content was maximum in the 6-week-old tissue induced with 3 ppm of Cr (7.63%) followed by 4 weeks tissue of tissue fed with 4 ppm of Ni (5.66%) and 4-week-old tissue fed with tyrosine 7.5 mg/100 ml (2.38%) among all samples used. These results suggest that tyrosine is better enhancer than Phe in the biosynthetic pathway of curculigoside. The presence of curculigoside in all extracts was confirmed by Fourier transform infrared spectroscopy, high-performance thin layer chromatography analysis with standard compound of curculigoside and histology of treated samples. CONCLUSION: This investigation was carried out for the 1(st) time, and it is a significant step in understanding the biochemistry of curculigoside. The developed protocol will be beneficial for marketing in pharmaceutical industries. SUMMARY: Curculigo orchioides Gaertn (Kali musli; Family: Hypoxidaceae) is an endangered medicinal plant used for many medicinal purposes such as impotency, aphrodisiac, tonic, jaundice, and skin ailments.It was observed that dry matter % was maximum in 6-week-old tissue fed with 2.5 mg/100 ml of tyrosine and diminished beyond this concentration among all samples usedThe nickel (Ni) and chromium (Cr) stress has enhanced the curculigoside in considerable amount in nontoxic range, in tissue culture of C. orchioides.Curculigoside Content was maximum in 6-week-old tissue induced with 3 ppm of Cr (7.63%; 11-fold enhancement) followed by 4 weeks tissue of tissue fed with 4 ppm of Ni (5.66%) and 4-week-old tissue fed with tyrosine 7.5 mg/100 ml (2.38%) among all samples used. Histological studies confirmed the enhanced production of curculigoside. Abbreviations Used: Phe: Phenylalanine, PAL: Phenylalanine ammonia-lyase, mM: mille Molar, Cr: Chromium, Ni: Nickel, HPTLC: High-performance thin layer chromatography.
Two new benzylbenzoate glucosides from Curculigo orchioides.[Pubmed:16814485]
Fitoterapia. 2006 Sep;77(6):416-9.
An extract from in vitro cultures of Curculigo orchioides grown as bulbils in shake flasks, afforded two new glucosides of substituted benzylbenzoate - Curculigoside C (3) and curculigoside D (4) - together with two known compounds - curculigoside A (1) and curculigoside B (2). Their structures were elucidated on the basis of spectral evidence, in particular by using 2D NMR methods. Their vasoactive properties were assessed in isolated rat aortic rings.
Antioxidative phenols and phenolic glycosides from Curculigo orchioides.[Pubmed:16079552]
Chem Pharm Bull (Tokyo). 2005 Aug;53(8):1065-7.
A new orcinol glucoside, orcinol-1-O-beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranoside (3), was isolated from the rhizomes of Curculigo orchioides GAERTN., together with seven known compounds: orcinol glucoside (1), orcinol-1-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranoside (2), curculigoside (4), curculigoside B (5), Curculigoside C (6), 2,6-dimethoxyl benzoic acid (7), and syringic acid (8). The structures of these compounds were elucidated using spectroscopic methods. The antioxidant activities of these isolated compounds were evaluated by colorimetric methods based on their scavenging effects on hydroxyl radicals and superoxide anion radicals, respectively. All the compounds showed potent antioxidative activities and the structure-activity relationship is discussed.


