OC000459Antagonist of D prostanoid receptor 2 ,potent and selective CAS# 851723-84-7, 950688-14-9 (sodium salt) |
- GW4064
Catalog No.:BCC4500
CAS No.:278779-30-9
- Chenodeoxycholic acid
Catalog No.:BCN2620
CAS No.:474-25-9
- XL335
Catalog No.:BCC4501
CAS No.:629664-81-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 851723-84-7, 950688-14-9 (sodium salt) | SDF | Download SDF |
| PubChem ID | 11462174 | Appearance | Powder |
| Formula | C21H17FN2O2 | M.Wt | 348.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
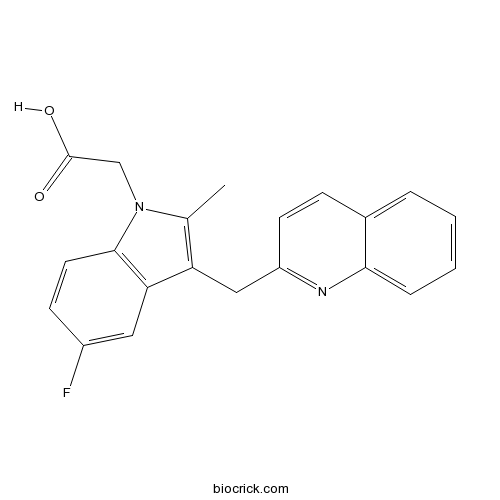
| Chemical Name | 2-[5-fluoro-2-methyl-3-(quinolin-2-ylmethyl)indol-1-yl]acetic acid | ||
| SMILES | CC1=C(C2=C(N1CC(=O)O)C=CC(=C2)F)CC3=NC4=CC=CC=C4C=C3 | ||
| Standard InChIKey | FATGTHLOZSXOBC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H17FN2O2/c1-13-17(11-16-8-6-14-4-2-3-5-19(14)23-16)18-10-15(22)7-9-20(18)24(13)12-21(25)26/h2-10H,11-12H2,1H3,(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | OC000459 is a potent and selective antagonist of D prostanoid receptor 2 (DP2) with an IC50 value of 13 nM. | |||||
| Targets | DP2 | hrCRTH2 | ||||
| IC50 | 13 nM | 13 nM (Ki) | ||||

OC000459 Dilution Calculator

OC000459 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8705 mL | 14.3526 mL | 28.7051 mL | 57.4102 mL | 71.7628 mL |
| 5 mM | 0.5741 mL | 2.8705 mL | 5.741 mL | 11.482 mL | 14.3526 mL |
| 10 mM | 0.2871 mL | 1.4353 mL | 2.8705 mL | 5.741 mL | 7.1763 mL |
| 50 mM | 0.0574 mL | 0.2871 mL | 0.5741 mL | 1.1482 mL | 1.4353 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.2871 mL | 0.5741 mL | 0.7176 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
OC000459 is a potent, selective and orally active antagonist of D prostanoid receptor 2 (DP2) with Ki value of 4nM [1].
DP2 is expressed on Th2 cells and eosinophils. It is activated by PGD2 which is reported to have effects on the pathogenesis of allergic disease. As an antagonist of DP2, OC000459 can prevent PGD2 from binding to human DP2 and rat recombinant DP2 with Ki values of 4nM and 3nM. In CHO cells expressing DP2, OC000459 suppresses PGD2 mediated calcium mobilization with IC50 value of 28nM. In human Th2 cells, OC000459 inhibits chemotaxis and production of IL-13 in response to PGD2 with IC50 values of 28nM and 19nM. In addition, oral administration of OC000459 effectively reduces blood eosinophilia in rats. OC000459 also shows inhibitory effect on the accumulation of eosinophil in guinea pigs aerosolized by DK-PGD2. Furthermore, OC000459 is also reported to inhibit the late-phase allergic response in patients with asthma [1, 2].
References:
[1] Pettipher R, Vinall S L, Xue L, et al. Pharmacologic profile of OC000459, a potent, selective, and orally active D prostanoid receptor 2 antagonist that inhibits mast cell-dependent activation of T helper 2 lymphocytes and eosinophils. Journal of Pharmacology and Experimental Therapeutics, 2012, 340(2): 473-482.
[2] Singh D, Cadden P, Hunter M, et al. Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459. European Respiratory Journal, 2013, 41(1): 46-52.
- Curculigoside C
Catalog No.:BCN3696
CAS No.:851713-74-1
- (S)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6600
CAS No.:85148-82-9
- Phospho-Glycogen Synthase Peptide-2 (substrate)
Catalog No.:BCC5747
CAS No.:851366-97-7
- PF9 tetrasodium salt
Catalog No.:BCC7854
CAS No.:851265-78-6
- 2-Methoxystypandrone
Catalog No.:BCN4400
CAS No.:85122-21-0
- THIP hydrochloride
Catalog No.:BCC6803
CAS No.:85118-33-8
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Cinchonain IIa
Catalog No.:BCN7716
CAS No.:85081-23-8
- NBI-98782
Catalog No.:BCC4277
CAS No.:85081-18-1
- BX517(PDK1 inhibitor2)
Catalog No.:BCC6391
CAS No.:850717-64-5
- GSK269962A
Catalog No.:BCC5178
CAS No.:850664-21-0
- PF 514273
Catalog No.:BCC7746
CAS No.:851728-60-4
- 3-(3-Chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepin-2-one
Catalog No.:BCC8587
CAS No.:85175-59-3
- ADX-47273
Catalog No.:BCC4598
CAS No.:851881-60-2
- RuBi-4AP
Catalog No.:BCC6044
CAS No.:851956-02-0
- TOK-001
Catalog No.:BCC3910
CAS No.:851983-85-2
- BAPTA
Catalog No.:BCC7483
CAS No.:85233-19-8
- 6-Methyl-7-O-methylaromadendrin
Catalog No.:BCN4010
CAS No.:852385-13-8
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
- Dovitinib Dilactic acid
Catalog No.:BCC3771
CAS No.:852433-84-2
- Futokadsurin C
Catalog No.:BCN6402
CAS No.:852459-91-7
Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis.[Pubmed:23379537]
Allergy. 2013 Mar;68(3):375-85.
BACKGROUND: Eosinophilic esophagitis (EoE) is a chronic, Th2-type inflammatory disease. Chemoattractant receptor-homologous molecule on Th2 cells (CRTH2) is a prostaglandin D(2) (PGD(2)) receptor, expressed by Th2 cells and other inflammatory cells, including eosinophils and basophils, that mediates chemotaxis and activation. OC000459 is a selective CRTH2 antagonist and would be expected to suppress eosinophilic tissue inflammation. The purpose of this study was to evaluate the efficacy and safety of an OC000459 monotherapy in adult patients with active, corticosteroid-dependent or corticosteroid-refractory EoE. METHODS: In this randomized, double-blind, placebo-controlled trial, 26 adult patients (m/f = 22/4; mean age 41 years, range 22-69 years) with active EoE, dependent or resistant to corticosteroids, were treated either with 100 mg OC000459 (n = 14) or placebo (n = 12) twice daily. Pre- and post-treatment disease activity was assessed clinically, endoscopically, histologically, and via biomarkers. The primary end point was the reduction in esophageal eosinophil infiltration. RESULTS: After an 8-week OC000459 treatment, the esophageal eosinophil load decreased significantly, from 114.83 to 73.26 eosinophils per high-power field [(eos/hpf), P = 0.0256], whereas no reduction was observed with placebo (102.80-99.47 eos/hpf, P = 0.870). With OC000459, the physician's global assessment of disease activity improved from 7.13 to 5.18 (P = 0.035). OC000459 likewise reduced extracellular deposits of eosinophil peroxidase and tenascin C, the effects not seen with placebo. No serious adverse events were observed. CONCLUSIONS: An 8-week treatment with the CRTH2-antagonist, OC000459, exerts modest, but significant, anti-eosinophil and beneficial clinical effects in adult patients with active, corticosteroid-dependent or corticosteroid-refractory EoE and is well tolerated.
The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial.[Pubmed:23025511]
Allergy. 2012 Dec;67(12):1572-9.
BACKGROUND: CRTH2 mediates activation of Th2 cells, eosinophils and basophils in response to prostaglandin D(2). The CRTH2 antagonist OC000459 has previously been demonstrated to reduce airway inflammation and improve lung function in moderate persistent asthma. The objective of the present study was to determine the involvement of CRTH2 in promoting nasal and ocular symptoms in allergic subjects exposed to grass pollen. METHODS: A single centre, randomised, double-blind, placebo-controlled, two-way crossover study was conducted in 35 male subjects allergic to grass pollen comparing OC000459 200 mg bid with placebo for 8 days. Subjects were exposed to grass pollen (>/= 1400 grains/m(3)) for 6 h on the 2nd and 8th days of treatment and assessed for nasal symptoms, ocular symptoms, other symptoms, nasal secretion weight and rhinomanometry over the 6-h period. After a washout period of 3 weeks, subjects were switched to the alternative treatment for a further 8 days. The trial was registered on the clinical trials.gov database (Identifier NCT01448902). RESULTS: During the first treatment period, treatment with OC000459 significantly reduced both nasal and ocular symptoms in allergic subjects compared with placebo after challenge with grass pollen. A significant effect was observed on the 2nd day of dosing which was increased on the 8th day of dosing. The therapeutic effects of OC000459 persisted into the second treatment period despite a 3-week washout phase. The safety profile of OC000459 was similar to that of placebo. CONCLUSION: Treatment with OC000459 was well tolerated and led to a significant and persistent reduction in the symptoms of rhinoconjunctivitis.
Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459.[Pubmed:22496329]
Eur Respir J. 2013 Jan;41(1):46-52.
CRTH2 (chemoattractant receptor expressed on T-helper (Th) type 2 cells) is a G-protein-coupled receptor expressed by Th2 lymphocytes and eosinophils that mediates prostaglandin (PG)D(2)-driven chemotaxis. We studied the efficacy of the oral CRTH2 antagonist OC000459 in steroid-naive asthmatic patients. A randomised, double-blind, placebo-controlled, two-way crossover study of 16 days' treatment with OC000459 (200 mg twice daily) on the late (LAR) and early (EAR) asthmatic responses to bronchial allergen challenge was conducted, with 16 subjects completing the study. There was a 25.4% (95% CI 5.1-45.6%) reduction in the LAR area under the curve (AUC) for change in forced expiratory volume in 1 s with OC000459 compared with placebo (p=0.018) but no effect on the EAR. Sputum eosinophil counts at 1 day post-allergen challenge were lower after OC000459 treatment (p=0.002). PGD(2)-induced blood eosinophil shape change ex vivo was assessed at day 7 (n=7). The AUC of eosinophil shift for OC000459 was lower than placebo; the mean difference was -33.6% (95% CI -66.8- -0.4%; p=0.048). OC000459 treatment inhibited LAR and post-allergen increase in sputum eosinophils. This CRTH2 antagonist appears to inhibit allergic inflammation in asthma.
Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459.[Pubmed:24866478]
Allergy. 2014 Sep;69(9):1223-32.
BACKGROUND: The CRTH2 antagonist OC000459 has previously been demonstrated to reduce airway inflammation and improve lung function in moderate persistent asthma. A study was conducted to determine the effect of lower once daily doses of OC000459 and to define the phenotype of subjects most responsive to treatment. METHODS: Adult subjects (percentage of predicted forced expiratory volume in 1 s (FEV1 ) 60-85%) were randomized to OC000459 at three dose levels (25 mg once daily, 200 mg once daily or 100 mg twice daily) or placebo for 12 weeks (n = 117-125 per group, full analysis set). The primary endpoint was the change from baseline in prebronchodilator FEV1 , and secondary endpoints included Asthma Control Questionnaire (ACQ) and Standardised Asthma Quality of Life Questionnaire [AQLQ(S)], and incidence of exacerbations and respiratory tract infections. RESULTS: OC459 caused a significant improvement in FEV1 compared with placebo at a dose of 25 mg once daily (P = 0.028). A similar increase was observed in the other dose groups, and the mean change in FEV1 in the pooled dose groups at endpoint was 95 ml greater than placebo (P = 0.024). In a post hoc analysis of atopic eosinophilic subjects with uncontrolled asthma, a mean increase in FEV1 of 220 ml was observed compared with placebo (P = 0.005). The mean increase in FEV1 was more marked in younger subjects in this group: for subjects aged OC000459. There were no drug-related serious adverse events. CONCLUSIONS: OC000459 is a safe and effective oral anti-inflammatory agent, which achieved clinically meaningful improvements in lung function and asthma control in allergic asthmatics with an eosinophil-dominant form of the disease. A dose of 25 mg given once daily was as effective as the higher doses studied.


