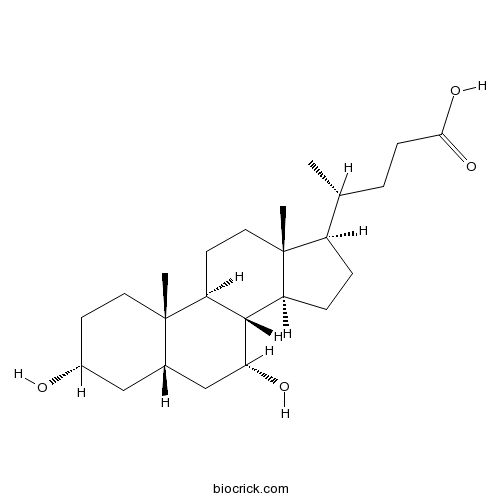
Chenodeoxycholic acidCAS# 474-25-9 |
- GW4064
Catalog No.:BCC4500
CAS No.:278779-30-9
- Obeticholic Acid
Catalog No.:BCC5572
CAS No.:459789-99-2
- XL335
Catalog No.:BCC4501
CAS No.:629664-81-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 474-25-9 | SDF | Download SDF |
| PubChem ID | 10133 | Appearance | Cryst. |
| Formula | C24H40O4 | M.Wt | 392.57 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | CDCA | ||
| Solubility | DMSO : ≥ 50 mg/mL (127.37 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (4R)-4-[(3R,5S,7R,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid | ||
| SMILES | CC(CCC(=O)O)C1CCC2C1(CCC3C2C(CC4C3(CCC(C4)O)C)O)C | ||
| Standard InChIKey | RUDATBOHQWOJDD-BSWAIDMHSA-N | ||
| Standard InChI | InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Chenodeoxycholic acid is a hydrophobic primary bile acid that activates nuclear receptors (FXR) involved in cholesterol metabolism. Chenodeoxycholic acid could reverse obesity in cHF-fed mice, mainly in response to the reduction in food intake. |
| Targets | IL Receptor | p38MAPK | PKA |
| In vitro | Acidic deoxycholic acid and chenodeoxycholic acid induce interleukin-8 production through p38 mitogen-activated protein kinase and protein kinase A in a squamous epithelial model.[Pubmed: 23425072]J Gastroenterol Hepatol. 2013 May;28(5):823-8.Immune-mediated mucosal inflammation characterized by the production of interleukin (IL)-8 is associated with the development of gastroesophageal reflux disease. The effects of bile acids, which are major components of reflux fluid, on the production of IL-8 and related mechanisms remain unclear. This study aimed to address these questions using an esophageal stratified squamous epithelial model.
|
| In vivo | Liver disease in infancy caused by oxysterol 7 α-hydroxylase deficiency: successful treatment with chenodeoxycholic acid.[Pubmed: 24658845]J Inherit Metab Dis. 2014 Sep;37(5):851-61.A child of consanguineous parents of Pakistani origin developed jaundice at 5 weeks and then, at 3 months, irritability, a prolonged prothrombin time, a low albumin, and episodes of hypoglycaemia. Investigation showed an elevated alanine aminotransferase with a normal γ-glutamyl-transpeptidase. Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans.[Pubmed: 23783097]J Clin Endocrinol Metab. 2013 Aug;98(8):3351-8.We hypothesized that intraduodenal infusions of Chenodeoxycholic acid (CDCA) would stimulate FGF and gut peptide secretion, thereby positively influencing glucose homeostasis. |
| Animal Research | Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice.[Pubmed: 24310401 ]Int J Obes (Lond). 2014 Aug;38(8):1027-34.In this study, the effects of another natural BA, Chenodeoxycholic acid (CDCA), on dietary obesity, UCP1 in both interscapular BAT and in white adipose tissue (brite cells in WAT), were characterized in dietary-obese mice. |

Chenodeoxycholic acid Dilution Calculator

Chenodeoxycholic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5473 mL | 12.7366 mL | 25.4732 mL | 50.9463 mL | 63.6829 mL |
| 5 mM | 0.5095 mL | 2.5473 mL | 5.0946 mL | 10.1893 mL | 12.7366 mL |
| 10 mM | 0.2547 mL | 1.2737 mL | 2.5473 mL | 5.0946 mL | 6.3683 mL |
| 50 mM | 0.0509 mL | 0.2547 mL | 0.5095 mL | 1.0189 mL | 1.2737 mL |
| 100 mM | 0.0255 mL | 0.1274 mL | 0.2547 mL | 0.5095 mL | 0.6368 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Chenodeoxycholic Acid (CDCA), is a hydrophobic primary bile acid that activates nuclear receptors(FXR) involved in cholesterol metabolism.
- Brazilin
Catalog No.:BCN5529
CAS No.:474-07-7
- Forskolin G
Catalog No.:BCN5527
CAS No.:473981-11-2
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Boc-Tyr(tBu)-OH
Catalog No.:BCC3462
CAS No.:47375-34-8
- SCH 563705
Catalog No.:BCC1933
CAS No.:473728-58-4
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- AMG 487
Catalog No.:BCC5140
CAS No.:473719-41-4
- Boc-Trp(For)-OH
Catalog No.:BCC3456
CAS No.:47355-10-2
- Carpinontriol B
Catalog No.:BCN8113
CAS No.:473451-73-9
- Spegatrine
Catalog No.:BCN4068
CAS No.:47326-53-4
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Tolbutamide Sodium
Catalog No.:BCC5632
CAS No.:473-41-6
- Citrostadienol
Catalog No.:BCN7357
CAS No.:474-40-8
- Reserpin N-oxide
Catalog No.:BCN3493
CAS No.:474-48-6
- Daucosterol
Catalog No.:BCN5531
CAS No.:474-58-8
- Campestanol
Catalog No.:BCN3890
CAS No.:474-60-2
- Campesterol
Catalog No.:BCN3181
CAS No.:474-62-4
- Brassicasterol
Catalog No.:BCN2613
CAS No.:474-67-9
- CAL-130 Racemate
Catalog No.:BCC1442
CAS No.:474012-90-3
- N-Methylsarpagine methosalt
Catalog No.:BCN5530
CAS No.:47418-70-2
- Monomethyl auristatin E
Catalog No.:BCC1775
CAS No.:474645-27-7
- 2,16-Kauranediol 2-O-beta-D-allopyranoside
Catalog No.:BCN1436
CAS No.:474893-07-7
- ST 91
Catalog No.:BCC7436
CAS No.:4749-61-5
- SB 657510
Catalog No.:BCC7713
CAS No.:474960-44-6
Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans.[Pubmed:23783097]
J Clin Endocrinol Metab. 2013 Aug;98(8):3351-8.
CONTEXT: Recent evidence suggests bile acids (BAs) are involved in the glycemic control via TGR5 activation with the subsequent release of gut peptides and farnesoid X receptor activation with ensuing release of fibroblast growth factors (FGFs). OBJECTIVE: We hypothesized that intraduodenal infusions of Chenodeoxycholic acid (CDCA) would stimulate FGF and gut peptide secretion, thereby positively influencing glucose homeostasis. DESIGN, SETTING, PARTICIPANTS, AND INTERVENTION: This randomized, double-blind, placebo-controlled, crossover trial included 12 healthy volunteers who received intraduodenal infusions (2.0 mL/min for 180 minutes) of saline, CDCA (5 or 15 mmol/L), and a fatty acid (sodium oleate), either alone or with 5 mmol/L CDCA. After 60 minutes, an oral glucose tolerance test (oGTT) was performed. MAIN OUTCOME MEASURES: Plasma levels of glucagon-like peptide-1 (GLP-1), peptide tyrosine tyrosine, cholecystokinin (CCK), total BAs, FGF19, FGF21, C-peptide, insulin, glucose, and glucagon were measured. RESULTS: Within the first 60 minutes, high-concentration CDCA induced a small but significant increase in GLP-1 and CCK secretion (P = .016 and P =.011), whereas plasma C-peptide, insulin, and glucose were not affected. Attenuated C-peptide and insulin release was observed after the oGTT with 15 mmol/L CDCA (P = .013 and P =.011). Plasma BA and FGF19 levels significantly increased after CDCA administration (P = .001 and P < .001). CONCLUSIONS: CDCA modulates GLP-1 and CCK secretion; the effect is small and does not influence glucose levels. The marked increase in plasma BAs and the attenuated insulin release after the oGTT indicate the role of BAs in glycemic control, independent of the incretin axis, and suggest involvement of farnesoid X receptor activation pathways.
Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice.[Pubmed:24310401]
Int J Obes (Lond). 2014 Aug;38(8):1027-34.
OBJECTIVE: Besides their role in lipid absorption, bile acids (BAs) can act as signalling molecules. Cholic acid was shown to counteract obesity and associated metabolic disorders in high-fat-diet (cHF)-fed mice while enhancing energy expenditure through induction of mitochondrial uncoupling protein 1 (UCP1) and activation of non-shivering thermogenesis in brown adipose tissue (BAT). In this study, the effects of another natural BA, Chenodeoxycholic acid (CDCA), on dietary obesity, UCP1 in both interscapular BAT and in white adipose tissue (brite cells in WAT), were characterized in dietary-obese mice. RESEARCH DESIGN: To induce obesity and associated metabolic disorders, male 2-month-old C57BL/6J mice were fed cHF (35% lipid wt wt(-1), mainly corn oil) for 4 months. Mice were then fed either (i) for 8 weeks with cHF or with cHF with two different doses (0.5%, 1%; wt wt(-1)) of CDCA (8-week reversion); or (ii) for 3 weeks with cHF or with cHF with 1% CDCA, or pair-fed (PF) to match calorie intake of the CDCA mice fed ad libitum; mice on standard chow diet were also used (3-week reversion). RESULTS: In the 8-week reversion, the CDCA intervention resulted in a dose-dependent reduction of obesity, dyslipidaemia and glucose intolerance, which could be largely explained by a transient decrease in food intake. The 3-week reversion revealed mild CDCA-dependent and food intake-independent induction of UCP1-mediated thermogenesis in interscapular BAT, negligible increase of UCP1 in subcutaneous WAT and a shift from carbohydrate to lipid oxidation. CONCLUSIONS: CDCA could reverse obesity in cHF-fed mice, mainly in response to the reduction in food intake, an effect probably occuring but neglected in previous studies using cholic acid. Nevertheless, CDCA-dependent and food intake-independent induction of UCP1 in BAT (but not in WAT) could contribute to the reduction in adiposity and to the stabilization of the lean phenotype.
Acidic deoxycholic acid and chenodeoxycholic acid induce interleukin-8 production through p38 mitogen-activated protein kinase and protein kinase A in a squamous epithelial model.[Pubmed:23425072]
J Gastroenterol Hepatol. 2013 May;28(5):823-8.
BACKGROUND AND AIM: Immune-mediated mucosal inflammation characterized by the production of interleukin (IL)-8 is associated with the development of gastroesophageal reflux disease. The effects of bile acids, which are major components of reflux fluid, on the production of IL-8 and related mechanisms remain unclear. This study aimed to address these questions using an esophageal stratified squamous epithelial model. METHODS: Normal human esophageal epithelial cells were seeded on the Transwell inserts and cultured with the air-liquid interface system to establish the model. Bile acids under different pH conditions were added to the apical compartment to examine their effects on IL-8 production and the underlying cellular signaling. RESULTS: Conjugated bile acids under a neutral or acidic condition did not induce IL-8 production, and unconjugated bile acids, deoxycholic acid (DCA), and Chenodeoxycholic acid (CDCA) all significantly induced IL-8 production, dose- and time-dependently, only under weakly acid conditions. Inhibition of p38 mitogen-activated protein kinase (p38 MAPK) and protein kinase A (PKA) attenuated the production of IL-8 induced by acidic DCA and CDCA. Inhibition of PKA did not block the bile acid-induced p38 MAPK activation. CONCLUSIONS: Compared with conjugated bile acids, the unconjugated bile acids DCA and CDCA are more likely to induce IL-8 production in vivo, especially under weakly acid conditions. This process involves two independent signaling pathways, p38 MAPK and PKA.
Liver disease in infancy caused by oxysterol 7 alpha-hydroxylase deficiency: successful treatment with chenodeoxycholic acid.[Pubmed:24658845]
J Inherit Metab Dis. 2014 Sep;37(5):851-61.
A child of consanguineous parents of Pakistani origin developed jaundice at 5 weeks and then, at 3 months, irritability, a prolonged prothrombin time, a low albumin, and episodes of hypoglycaemia. Investigation showed an elevated alanine aminotransferase with a normal gamma-glutamyl-transpeptidase. Analysis of urine by electrospray ionisation tandem mass spectrometry (ESI-MS/MS) showed that the major peaks were m/z 480 (taurine-conjugated 3beta-hydroxy-5-cholenoic acid) and m/z 453 (sulphated 3beta-hydroxy-5-cholenoic acid). Analysis of plasma by gas chromatography-mass spectrometry (GC-MS) showed increased concentrations of 3beta-hydroxy-5-cholenoic acid, 3beta-hydroxy-5-cholestenoic acid and 27-hydroxycholesterol, indicating oxysterol 7 alpha-hydroxylase deficiency. The patient was homozygous for a mutation (c.1249C>T) in CYP7B1 that alters a highly conserved residue in oxysterol 7 alpha-hydroxylase (p.R417C) - previously reported in a family with hereditary spastic paraplegia type 5. On treatment with ursodeoxycholic acid (UDCA), his condition was worsening, but on Chenodeoxycholic acid (CDCA), 15 mg/kg/d, he improved rapidly. A biopsy (after 2 weeks on CDCA), showed a giant cell hepatitis, an evolving micronodular cirrhosis, and steatosis. The improvement in liver function on CDCA was associated with a drop in the plasma concentrations and urinary excretions of the 3beta-hydroxy-Delta5 bile acids which are considered hepatotoxic. At age 5 years (on CDCA, 6 mg/kg/d), he was thriving with normal liver function. Neurological development was normal apart from a tendency to trip. Examination revealed pes cavus but no upper motor neuron signs. The findings in this case suggest that CDCA can reduce the activity of cholesterol 27-hydroxylase - the first step in the acidic pathway for bile acid synthesis.


