Tolbutamide SodiumCAS# 473-41-6 |
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- ML133 HCl
Catalog No.:BCC5006
CAS No.:1222781-70-5
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Nicorandil
Catalog No.:BCC5004
CAS No.:65141-46-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 473-41-6 | SDF | Download SDF |
| PubChem ID | 87651982 | Appearance | Powder |
| Formula | C12H17N2NaO3S | M.Wt | 292.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
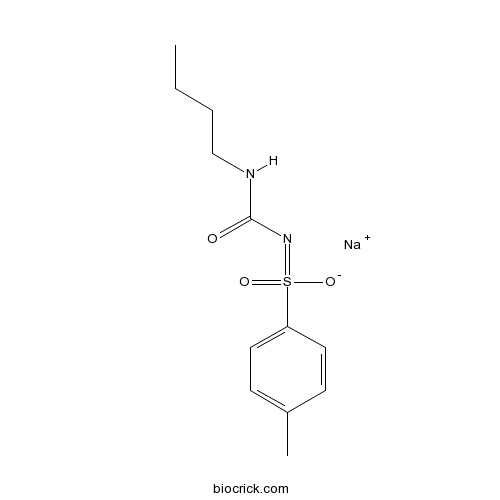
| Chemical Name | sodium;N-(butylcarbamoyl)-4-methylbenzenesulfonimidate | ||
| SMILES | CCCCNC(=O)N=S(=O)(C1=CC=C(C=C1)C)[O-].[Na+] | ||
| Standard InChIKey | LPVSVPABNXNFKY-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C12H18N2O3S.Na/c1-3-4-9-13-12(15)14-18(16,17)11-7-5-10(2)6-8-11;/h5-8H,3-4,9H2,1-2H3,(H2,13,14,15,16,17);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tolbutamide Sodium Dilution Calculator

Tolbutamide Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4208 mL | 17.104 mL | 34.2079 mL | 68.4158 mL | 85.5198 mL |
| 5 mM | 0.6842 mL | 3.4208 mL | 6.8416 mL | 13.6832 mL | 17.104 mL |
| 10 mM | 0.3421 mL | 1.7104 mL | 3.4208 mL | 6.8416 mL | 8.552 mL |
| 50 mM | 0.0684 mL | 0.3421 mL | 0.6842 mL | 1.3683 mL | 1.7104 mL |
| 100 mM | 0.0342 mL | 0.171 mL | 0.3421 mL | 0.6842 mL | 0.8552 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- beta-Eudesmol
Catalog No.:BCN6294
CAS No.:473-15-4
- alpha-Cyperone
Catalog No.:BCN1193
CAS No.:473-08-5
- SB 366791
Catalog No.:BCC7128
CAS No.:472981-92-3
- 8(14),15-Isopimaradien-3-ol
Catalog No.:BCN5526
CAS No.:4728-30-7
- 1-Benzyl-4-hydroxypiperidine
Catalog No.:BCC8459
CAS No.:4727-72-4
- Kartogenin
Catalog No.:BCC6211
CAS No.:4727-31-5
- H-Ser(Bzl)-OH
Catalog No.:BCC3031
CAS No.:4726-96-9
- Astaxanthin
Catalog No.:BCN2248
CAS No.:472-61-7
- Masticadienolic acid
Catalog No.:BCN5525
CAS No.:472-30-0
- Butyrospermol
Catalog No.:BCN3340
CAS No.:472-28-6
- Telocinobufagin
Catalog No.:BCN2359
CAS No.:472-26-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Spegatrine
Catalog No.:BCN4068
CAS No.:47326-53-4
- Carpinontriol B
Catalog No.:BCN8113
CAS No.:473451-73-9
- Boc-Trp(For)-OH
Catalog No.:BCC3456
CAS No.:47355-10-2
- AMG 487
Catalog No.:BCC5140
CAS No.:473719-41-4
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- SCH 563705
Catalog No.:BCC1933
CAS No.:473728-58-4
- Boc-Tyr(tBu)-OH
Catalog No.:BCC3462
CAS No.:47375-34-8
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Forskolin G
Catalog No.:BCN5527
CAS No.:473981-11-2
- Brazilin
Catalog No.:BCN5529
CAS No.:474-07-7
- Chenodeoxycholic acid
Catalog No.:BCN2620
CAS No.:474-25-9
Tenidap sodium does not alter the clearance or plasma protein binding of tolbutamide in healthy male volunteers.[Pubmed:7547093]
Br J Clin Pharmacol. 1995;39 Suppl 1:39S-42S.
1. This randomised, double-blind, parallel group study in 12 healthy young men compared the effects of tenidap sodium 120 mg day-1, at steady state, with placebo on the plasma protein binding and clearance of tolbutamide. 2. Each subject received a 1000 mg intravenous infusion of tolbutamide given over 5 min on day 1 of the study, and again on day 30 following 22 days of successive tenidap or placebo administration. 3. The percentage of unbound tolbutamide in plasma was determined immediately before each infusion. Mean pharmacokinetic parameters (system plasma clearance, terminal phase rate constant, apparent volume of distribution at steady state) of tolbutamide were derived from individual tolbutamide plasma concentration-time curves generated after infusion. The within group day 30 minus day 1 differences were compared between treatment groups. 4. Tenidap was shown to have no statistically or clinically significant effects on any of the parameters assessed. These results indicate that tenidap does not induce or inhibit the P450IIC9 isozyme which metabolises tolbutamide and that tenidap does not displace tolbutamide from plasma protein binding sites. 5. Both tenidap and tolbutamide were well tolerated. No severe treatment-related adverse events were reported, no subject withdrew from the study, and there were no reports of treatment-related laboratory abnormalities, or significant variations in vital signs.
Tolbutamide perifusion of rat islets. Sequential changes in calcium, phosphorus, sodium, potassium, and chlorine in single beta cells.[Pubmed:6348090]
J Clin Invest. 1983 Aug;72(2):478-82.
Fluctuations of calcium, phosphorus, sodium, potassium, and chlorine in beta cells were followed during rat islet perifusion with tolbutamide and related to insulin secretion. In 24 paired experiments two chambers containing 100 islets were perifused with buffered medium containing 4.2 mM glucose alone or with added tolbutamide (200 micrograms/ml). Effluent was collected frequently for insulin determinations. At eight different time intervals from 0 to 20 min islets were acutely fixed, prepared for scanning electron microscopy and beta cells in islet tissue were identified. Element content in 480 single cells was measured by energy dispersive x-ray analysis. Tolbutamide elicited typical monophasic insulin release that exceeded control islet secretory rates from 2 to 6 min with a peak value at 3 min. This pattern was preceded by monophasic calcium accumulation in beta cells that abruptly rose 150% above control cells at 1 min and declined to base line by 4 min. The rapid ascent of calcium was associated with significant depressions of sodium and potassium content without alterations of cell phosphorus. Chlorine fell at 2 min and then rose greater than 50% above control cells at 4 min. After 6 min insulin secretion and element content remained near control levels. We conclude that monophasic calcium accumulation in beta cells is the earliest, most predictive event of islet insulin secretion after a tolbutamide stimulus. Oscillations of beta cell sodium and potassium reciprocally relate to calcium, and an elevation of chlorine content is a relatively late phenomenon in the stimulus-secretion coupling process.
Opposing effects of glucose and tolbutamide on the sodium content of rat pancreatic islets.[Pubmed:3291536]
Acta Endocrinol (Copenh). 1988 Jun;118(2):227-31.
Integrating flame photometry was employed for measuring sodium in rat pancreatic islets incubated in media buffered with HEPES or bicarbonate. The sodium content decreased by nearly 40% when the islets were exposed to 5 mmol/l glucose, no further reduction being seen with additional rise of the concentration to 20 mmol/l. Whereas the depressing effect of glucose was mimicked by 100 mumol/l quinine, increased sodium contents were noted after inhibition of the Na/K pump (removal of extracellular K+ or addition of 1 mmol/l ouabain) or exposure of the islets to 1 mmol/l tolbutamide. Although promoting sodium accumulation in the islet cells, tolbutamide counteracted the increase in sodium obtained on withdrawal of K+ from the incubation medium. It is suggested that tolbutamide in addition to its major effect in promoting the entry of Ca2+ also facilitates insulin release by suppressing the outward transport of this ion.
Free and bound sodium in pancreatic beta-cells exposed to glucose and tolbutamide.[Pubmed:2679551]
Biochem Biophys Res Commun. 1989 Oct 16;164(1):212-8.
The effects of glucose and tolbutamide on the sodium handling of the pancreatic beta-cells were evaluated by measuring the total sodium content in intact islets from ob/ob-mice by integrating flame photometry and the free ion in individual beta-cells by dual wavelength fluorometry. Whereas increasing the glucose concentration from 3 to 20 mM resulted in a lowering of sodium, the addition of 100 microM tolbutamide caused a rise. The above-mentioned effects were most marked (about 50%) for the physiologically significant free sodium. The data indicate a more important role for Na+ in the regulation of insulin release than so far acknowledged. Increase of Na+ may contribute to the secretory response to hypoglycemic sulfonylureas by providing an additional rise of cytoplasmic Ca2+.


