KartogeninPotently induces chondrogenesis in MSCs CAS# 4727-31-5 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4727-31-5 | SDF | Download SDF |
| PubChem ID | 2826191 | Appearance | Powder |
| Formula | C20H15NO3 | M.Wt | 317.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | KGN | ||
| Solubility | DMSO : ≥ 42 mg/mL (132.35 mM) *"≥" means soluble, but saturation unknown. | ||
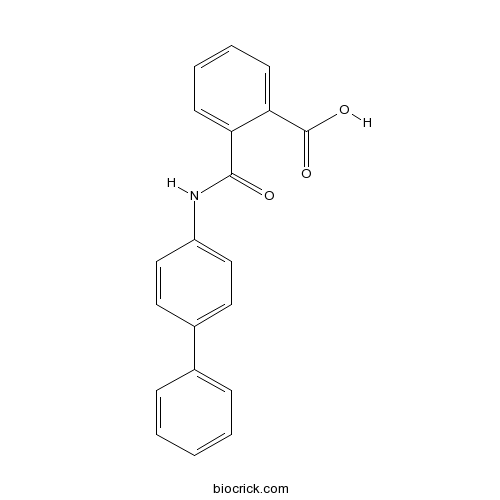
| Chemical Name | 2-[(4-phenylphenyl)carbamoyl]benzoic acid | ||
| SMILES | C1=CC=C(C=C1)C2=CC=C(C=C2)NC(=O)C3=CC=CC=C3C(=O)O | ||
| Standard InChIKey | SLUINPGXGFUMLL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H15NO3/c22-19(17-8-4-5-9-18(17)20(23)24)21-16-12-10-15(11-13-16)14-6-2-1-3-7-14/h1-13H,(H,21,22)(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potently induces differentiation of human mesenchymal stem cells into chondrocytes (EC50 = 100 nM). Reduces disease severity in a mouse model of osteoarthritis; displays protective effects against osteoarthritic stimuli in mature chondrocytes in vitro. |

Kartogenin Dilution Calculator

Kartogenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1512 mL | 15.756 mL | 31.5119 mL | 63.0239 mL | 78.7799 mL |
| 5 mM | 0.6302 mL | 3.1512 mL | 6.3024 mL | 12.6048 mL | 15.756 mL |
| 10 mM | 0.3151 mL | 1.5756 mL | 3.1512 mL | 6.3024 mL | 7.878 mL |
| 50 mM | 0.063 mL | 0.3151 mL | 0.6302 mL | 1.2605 mL | 1.5756 mL |
| 100 mM | 0.0315 mL | 0.1576 mL | 0.3151 mL | 0.6302 mL | 0.7878 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Kartogenin is an inducer of differentiation of human mesenchymal stem cells into chondrocytes.
In Vitro:Kartogenin enhances cell proliferation in both cell types in a concentration-dependent manner and induces chondrogenic differentiation of stem cells, as demonstrated by high expression levels of chondrogenic markers aggrecan, collagen II and Sox-9. Besides, kartogenin induces the formation of cartilage-like tissues in cell cultures, as observed through the staining of abundant proteoglycans, collagen II and osteocalcin[1]. Kartogenin stimulates type-I collagen synthesis of fibroblasts at the mRNA and protein levels in a time-dependent manner without obvious influence on fibroblasts’ apoptosis and viability. Smad4/smad5 of the TGF-β signaling pathway is activated by kartogenin while MAPK signaling pathway remains unchanged[2]. Kartogenin treatment enhances chondrocyte pericellular matrix assembly and retention in the presence of IL-1β. Kartogenin partially blocks the IL-1β-induced increased expression of ADAMTS-5. Additionally, kartogenin-treated articular chondrocytes exhibits a decrease in CD44 proteolytic fragmentation[3].
In Vivo:hen injected into intact rat patellar tendons, kartogenin induces cartilage-like tissue formation in the injected area. When injected into experimentally injured rat Achilles TBJs, wound healing in the TBJs is enhanced, as evidenced by the formation of extensive cartilage-like tissues[1]. Kartogenin stimulates collagen synthesis in the mouse dermis. Dermis in the kartogenin (100 nM)-treated group exhibits increased dermal thickness and intense blue staining, which represents more collagen composition in the dermis[2].
References:
[1]. Zhang J, et al. Kartogenin induces cartilage-like tissue formation in tendon-bone junction. Bone Res. 2014;2. pii: 14008.
[2]. Wang J, et al. A heterocyclic molecule kartogenin induces collagen synthesis of human dermal fibroblasts by activating the smad4/smad5 pathway. Biochem Biophys Res Commun. 2014 Jul 18;450(1):568-74.
[3]. Ono Y, et al. Chondroprotective Effect of Kartogenin on CD44-Mediated Functions in Articular Cartilage and Chondrocytes. Cartilage. 2014 Jul;5(3):172-80.
- H-Ser(Bzl)-OH
Catalog No.:BCC3031
CAS No.:4726-96-9
- Astaxanthin
Catalog No.:BCN2248
CAS No.:472-61-7
- Masticadienolic acid
Catalog No.:BCN5525
CAS No.:472-30-0
- Butyrospermol
Catalog No.:BCN3340
CAS No.:472-28-6
- Telocinobufagin
Catalog No.:BCN2359
CAS No.:472-26-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Ruscogenin
Catalog No.:BCN6287
CAS No.:472-11-7
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- Boc-D-Ser(Bzl)-OH
Catalog No.:BCC3448
CAS No.:47173-80-8
- (E)-Aldosecologanin
Catalog No.:BCN4631
CAS No.:471271-55-3
- Bufotaline
Catalog No.:BCN5368
CAS No.:471-95-4
- Stachydrine
Catalog No.:BCN8384
CAS No.:471-87-4
- 1-Benzyl-4-hydroxypiperidine
Catalog No.:BCC8459
CAS No.:4727-72-4
- 8(14),15-Isopimaradien-3-ol
Catalog No.:BCN5526
CAS No.:4728-30-7
- SB 366791
Catalog No.:BCC7128
CAS No.:472981-92-3
- alpha-Cyperone
Catalog No.:BCN1193
CAS No.:473-08-5
- beta-Eudesmol
Catalog No.:BCN6294
CAS No.:473-15-4
- Tolbutamide Sodium
Catalog No.:BCC5632
CAS No.:473-41-6
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Spegatrine
Catalog No.:BCN4068
CAS No.:47326-53-4
- Carpinontriol B
Catalog No.:BCN8113
CAS No.:473451-73-9
- Boc-Trp(For)-OH
Catalog No.:BCC3456
CAS No.:47355-10-2
- AMG 487
Catalog No.:BCC5140
CAS No.:473719-41-4
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
Hyaluronic Acid Hydrogel Functionalized with Self-Assembled Micelles of Amphiphilic PEGylated Kartogenin for the Treatment of Osteoarthritis.[Pubmed:28338415]
Tissue Eng Part A. 2017 Jul;23(13-14):630-639.
Synthetic hyaluronic acid (HA) containing a covalently integrated drug is capable of releasing therapeutic molecules and is an attractive candidate for the intra-articular treatment of osteoarthritis (OA). Herein, self-assembled PEGylated Kartogenin (PEG/KGN) micelles consisting of hydrophilic polyethylene glycol (PEG) and hydrophobic KGN, which has been shown to induce chondrogenesis in human mesenchymal stem cells, were prepared by covalent crosslinking. HA hydrogels containing PEG/KGN micelles (HA/PEG/KGN) were prepared by covalently bonding PEG chains to HA. The physicochemical properties of the HA/PEG/KGN conjugate gels were investigated using Fourier transform infrared spectroscopy, (1)H NMR, dynamic light scattering (DLS), and scanning electron microscopy (SEM). HA/PEG/KGN gels exhibited larger micelles in aqueous solution than PEG/KGN. SEM images of PEG/KGN micelles showed a dark core and a bright shell, whereas PEG/KGN micelles covalently integrated into HA had an irregular oval shape. Covalent integration of PEG/KGN micelles in HA hydrogels significantly reduced drug release rates and provided sustained release over a prolonged period of time. HA/PEG/KGN hydrogels were degradable enzymatically by collagenase and hyaluronidase in vitro. Injection of HA/PEG/KGN hydrogels into articular cartilage significantly suppressed the progression of OA in rats compared with free-HA hydrogel injection. These results suggest that the HA/PEG/KGN hydrogels have greater potency than free-HA hydrogels against OA as biodegradable synthetic therapeutics.
Modulation of Superficial Zone Protein/Lubricin/PRG4 by Kartogenin and Transforming Growth Factor-beta1 in Surface Zone Chondrocytes in Bovine Articular Cartilage.[Pubmed:27688846]
Cartilage. 2016 Oct;7(4):388-97.
OBJECTIVE: Superficial zone protein (SZP)/lubricin/PRG4 functions as a boundary lubricant in articular cartilage to decrease friction and wear. As articular cartilage lubrication is critical for normal joint function, the accumulation of SZP at the surface of cartilage is important for joint homeostasis. Recently, a heterocyclic compound called Kartogenin (KGN) was found to induce chondrogenic differentiation and enhance mRNA expression of lubricin. The objective of this study was to determine whether KGN can stimulate synthesis of SZP in superficial zone, articular chondrocytes. DESIGN: We investigated the effects of KGN and transforming growth factor-beta1 (TGF-beta1) on articular cartilage and synovium of the bovine knee joint by evaluating SZP secretion by enzyme-linked immunosorbent assay analysis. Monolayer, micromass, and explant cultures of articular cartilage, and monolayer culture of synoviocytes, were treated with KGN. SZP accumulation in the medium was evaluated and mRNA expression was measured through quantitative polymerase chain reaction. RESULTS: TGF-beta1 stimulated SZP secretion by superficial zone chondrocytes in monolayer, explant, and micromass cultures as expected. In addition, SZP secretion was inhibited by IL-1beta in explant cultures, and enhanced by TGF-beta1 in synoviocyte monolayer cultures. Although KGN elicited a 1.2-fold increase in SZP mRNA expression in combination with TGF-beta1, KGN neither stimulated any significant increases in SZP synthesis nor prevented catabolic decreases in SZP production from IL-1beta. CONCLUSIONS: These data suggest that the chondrogenic effects of KGN depend on cellular phenotype and differentiation status, as KGN did not alter SZP synthesis in differentiated, superficial zone articular chondrocytes.
Development of kartogenin-conjugated chitosan-hyaluronic acid hydrogel for nucleus pulposus regeneration.[Pubmed:28261733]
Biomater Sci. 2017 Mar 28;5(4):784-791.
Injectable constructs for in vivo gelation have many advantages in the regeneration of degenerated nucleus pulposus. In this study, an injectable hydrogel consisting of chitosan (CS) and hyaluronic acid (HA) crosslinked with glycerol phosphate (GP) at different proportions (CS : GP : HA, 6 : 3 : 1, 5 : 3 : 2, 4 : 3 : 3, 3 : 3 : 4, 2 : 3 : 5, 1 : 3 : 6, V : V : V) was developed and employed as a delivery system for Kartogenin (KGN), a biocompound that can activate chondrocytes. In vitro gelation time, morphologies, swelling, weight loss, compressive modulus and cumulative release of KGN in hydrogels were studied. For biocompatibility assessments, human adipose-derived stem cells (ADSCs) were encapsulated in these hydrogels. The effects of KGN on stem cell proliferation and differentiation into nucleus pulposus-like cells were examined. The hydrogels with higher concentrations of HA showed a slightly shorter gelation time, higher water uptake, faster weight loss and faster KGN release compared to the hydrogels with lower concentrations of HA. As the KGN-conjugated hydrogel prepared with the proportions 5 : 3 : 2 displayed good mechanical properties, it was chosen as the optimal gel to promote cell proliferation and differentiation. No significant difference was seen in the expression levels of nucleus pulposus markers induced by KGN or TGF-beta. Additionally, inclusion of KGN and TGF-beta together did not produce a synergistic effect in inducing nucleus pulposus properties. In conclusion, we have developed a KGN-conjugated CS/HA hydrogel (5 : 3 : 2) with sustained release of KGN in hydrogel that can promote ADSC proliferation and nucleus pulposus differentiation. This kind of hydrogel may be a simple and effective candidate for the repair of degenerative NP tissue after minimally invasive surgery.
Kartogenin with PRP promotes the formation of fibrocartilage zone in the tendon-bone interface.[Pubmed:28127950]
J Tissue Eng Regen Med. 2017 Dec;11(12):3445-3456.
Treatment of tendon-bone junction injuries is a challenge because tendon-bone interface often heals poorly and the fibrocartilage zone, which reduces stress concentration, at the interface is not formed. In this study, we used a compound called Kartogenin (KGN) with platelet-rich plasma (PRP) to induce the formation of fibrocartilage zone in a rat tendon graft-bone tunnel model. The experimental rats received KGN-PRP or PRP injections in the tendon graft-bone tunnel interface. The control group received saline. After 4, 8 and 12 weeks, Safranin O staining of the tendon graft-bone tunnels revealed abundant proteoglycans in the KGN-PRP group indicating the formation of cartilage-like transition zone. Immunohistochemical and immuno-fluorescence staining revealed collagen types I (Col-I) and II (Col-II) in the newly formed fibrocartilage zone. Both fibrocartilage zone formation and maturation were healing time dependent. In contrast, the PRP and saline control groups had no cartilage-like tissues and minimal Col-I and Col-II staining. Some gaps were also present in the saline control group. Finally, pull-out strength in the KGN-PRP-treated group at 8 weeks was 1.4-fold higher than the PRP-treated group and 1.6-fold higher than the saline control group. These findings indicate that KGN, with PRP as a carrier, promotes the formation of fibrocartilage zone between the tendon graft and bone interface. Thus, KGN-PRP may be used as a convenient cell-free therapy in clinics to promote fibrocartilage zone formation in rotator calf repair and anterior cruciate ligament reconstruction, thereby enhancing the mechanical strength of the tendon-bone interface and hence the clinical outcome of these procedures. Copyright (c) 2017 John Wiley & Sons, Ltd.
A stem cell-based approach to cartilage repair.[Pubmed:22491093]
Science. 2012 May 11;336(6082):717-21.
Osteoarthritis (OA) is a degenerative joint disease that involves the destruction of articular cartilage and eventually leads to disability. Molecules that promote the selective differentiation of multipotent mesenchymal stem cells (MSCs) into chondrocytes may stimulate the repair of damaged cartilage. Using an image-based high-throughput screen, we identified the small molecule Kartogenin, which promotes chondrocyte differentiation (median effective concentration = 100 nM), shows chondroprotective effects in vitro, and is efficacious in two OA animal models. Kartogenin binds filamin A, disrupts its interaction with the transcription factor core-binding factor beta subunit (CBFbeta), and induces chondrogenesis by regulating the CBFbeta-RUNX1 transcriptional program. This work provides new insights into the control of chondrogenesis that may ultimately lead to a stem cell-based therapy for osteoarthritis.


