StachydrineCAS# 471-87-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 471-87-4 | SDF | Download SDF |
| PubChem ID | 115244 | Appearance | White crystalline powder |
| Formula | C7H13NO2 | M.Wt | 143.18 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 32 mg/mL (223.49 mM) DMSO : ≥ 26 mg/mL (181.59 mM) *"≥" means soluble, but saturation unknown. | ||
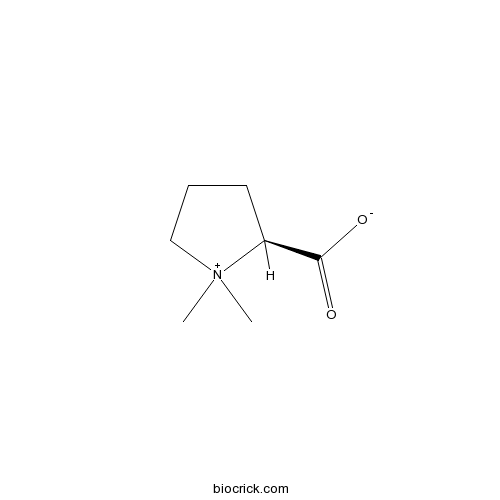
| Chemical Name | (2S)-1,1-dimethylpyrrolidin-1-ium-2-carboxylate | ||
| SMILES | C[N+]1(CCCC1C(=O)[O-])C | ||
| Standard InChIKey | CMUNUTVVOOHQPW-LURJTMIESA-N | ||
| Standard InChI | InChI=1S/C7H13NO2/c1-8(2)5-3-4-6(8)7(9)10/h6H,3-5H2,1-2H3/t6-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Stachydrine Dilution Calculator

Stachydrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.9842 mL | 34.9211 mL | 69.8422 mL | 139.6843 mL | 174.6054 mL |
| 5 mM | 1.3968 mL | 6.9842 mL | 13.9684 mL | 27.9369 mL | 34.9211 mL |
| 10 mM | 0.6984 mL | 3.4921 mL | 6.9842 mL | 13.9684 mL | 17.4605 mL |
| 50 mM | 0.1397 mL | 0.6984 mL | 1.3968 mL | 2.7937 mL | 3.4921 mL |
| 100 mM | 0.0698 mL | 0.3492 mL | 0.6984 mL | 1.3968 mL | 1.7461 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Stachydrine is a major constituent of Chinese herb leonurus heterophyllus sweet used to promote blood circulation and dispel blood stasis. Stachydrine can inhibit the NF-κB signal pathway.
In Vitro:Stachydrine can inhibit the NF-κB signal pathway, and this may be related to the mechanism of anti-hypertrophic. Intervention of stachydrine significantly suppresses the level of p-IκB protein in the cytosol and NF-κB protein in the nucleus [1]. Tissue factor mRNA is decreased in stachydrine-treated human umbilical vein endothelial cells. Stachydrine attenuates the decline of human umbilical vein endothelial cells viability and the increase of LDH activity induced by anoxia-reoxygenation[2]. A dose dependent decrease in expression of mRNA, and protein levels are observed in stachydrine-treated human prostate cancer cells (PC-3 and LNcaP)[3].
In Vivo:Stachydrine attenuates norepinephrine-induced cardiomyocyte hypertrophy and has potential protective effects against β-adrenergic receptor induced Ca2+ mishandling[4]. Stachydrine treatment reduces the expressions of PERK, CHOP, and caspase-3 in the endoplasmic reticulum stress-related apoptosis pathway[5].
References:
[1]. Guo W, et al. Effect of Leonurus stachydrine on myocardial cell hypertrophy. Zhong Yao Cai. 2012 Jun;35(6):940-3.
[2]. Yin J, et al. Stachydrine, a major constituent of the Chinese herb leonurus heterophyllus sweet, ameliorates human umbilical vein endothelial cells injury induced by anoxia-reoxygenation. Am J Chin Med. 2010;38(1):157-71.
[3]. Rathee P, et al. In vitro anticancer activity of stachydrine isolated from Capparis decidua on prostate cancer cell lines. Nat Prod Res. 2012;26(18):1737-40.
[4]. Zhang C, et al. Effects of stachydrine on norepinephrine-induced neonatal rat cardiac myocytes hypertrophy and intracellular calcium transients. BMC Complement Altern Med. 2014 Dec 8;14:474.
[5]. Zhang C, et al. Effect of stachydrine on endoplasmic reticulum stress-induced apoptosis in rat kidney after unilateral ureteral obstruction. J Asian Nat Prod Res. 2013;15(4):373-81.
- (-)-Steviol
Catalog No.:BCN8358
CAS No.:471-80-7
- Dipterocarpol
Catalog No.:BCN5523
CAS No.:471-69-2
- alpha-Boswellic acid
Catalog No.:BCN5522
CAS No.:471-66-9
- Isocolumbin
Catalog No.:BCN5361
CAS No.:471-54-5
- Glycyrrhetinic acid
Catalog No.:BCN5942
CAS No.:471-53-4
- 8-Amino-7-oxononanoic acid
Catalog No.:BCN1778
CAS No.:4707-58-8
- Atraric acid
Catalog No.:BCN5521
CAS No.:4707-47-5
- alpha-Lapachone
Catalog No.:BCN5520
CAS No.:4707-33-9
- Beta-Lapachone
Catalog No.:BCC5088
CAS No.:4707-32-8
- Benzoyl-DL-methionine
Catalog No.:BCC8863
CAS No.:4703-38-2
- Cineole
Catalog No.:BCN2686
CAS No.:470-82-6
- 1-Kestose
Catalog No.:BCN8292
CAS No.:470-69-9
- Bufotaline
Catalog No.:BCN5368
CAS No.:471-95-4
- (E)-Aldosecologanin
Catalog No.:BCN4631
CAS No.:471271-55-3
- Boc-D-Ser(Bzl)-OH
Catalog No.:BCC3448
CAS No.:47173-80-8
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- Ruscogenin
Catalog No.:BCN6287
CAS No.:472-11-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Telocinobufagin
Catalog No.:BCN2359
CAS No.:472-26-4
- Butyrospermol
Catalog No.:BCN3340
CAS No.:472-28-6
- Masticadienolic acid
Catalog No.:BCN5525
CAS No.:472-30-0
- Astaxanthin
Catalog No.:BCN2248
CAS No.:472-61-7
- H-Ser(Bzl)-OH
Catalog No.:BCC3031
CAS No.:4726-96-9
- Kartogenin
Catalog No.:BCC6211
CAS No.:4727-31-5
Stachydrine ameliorates carbon tetrachloride-induced hepatic fibrosis by inhibiting inflammation, oxidative stress and regulating MMPs/TIMPs system in rats.[Pubmed:29378386]
Biomed Pharmacother. 2018 Jan;97:1586-1594.
Inflammation and oxidative stress are two crucial factors mediating liver fibrosis. Stachydrine (STA) is a naturally occurring compound extracted from a medicinal plant Leonuru heterophyllus, which can inhibit the proliferation and induce the apoptosis of breast cancer cells, relieve high glucose-induced endothelial cell senescence and isoproterenol-induced cardiac hypertrophy, and exert antitumor effects. However, its roles in hepatic fibrosis remain largely unknown. We aimed to evaluate the effect of STA on carbon tetrachloride (CCl4)-induced hepatic fibrosis in rats and to elucidate the possible mechanisms. STA alleviated the pathological changes caused by CCl4 injection in livers compared to the normal liver. Hematoxylin-eosin staining further showed that STA treatment remarkably improved the liver histology, as evidenced by mitigated hepatic steatosis, necrosis, and fibrotic septa. STA reduced the liver/body weight ratio and the serum levels of aminotransferase, aspartate aminotransferase and alkaline phosphatase. It also significantly decreased collagen deposition and hydroxyproline level. Both mRNA and protein levels of alpha-SMA, alpha1(I)-procollagen and fibronectin were decreased by STA compared to those of the model group. STA significantly inhibited the expressions of inflammatory factors interleukin-6 (IL-6), IL-8, IL-1beta, tumor necrosis factor-alpha, inducible nitric oxide synthase and cyclooxygenase-2. It suppressed oxidative stress by decreasing malondialdehyde level as well as increasing glutathione level and enzymatic activities of superoxide dismutase, catalase, glutathione reductase and glutathione peroxidase. STA also significantly increased the protein expressions of tissue inhibitor of metallopeptidase-1 (TIMP-1) and TIMP-2 but decreased those of matrix metalloproteinase-2 (MMP-2) and MMP-9, indicating excessive basement membrane in the fibrotic liver. Collectively, STA has potent protective effects on the liver, with therapeutic implication for liver fibrosis.
Angiogenic effect of motherwort (Leonurus japonicus) alkaloids and toxicity of motherwort essential oil on zebrafish embryos.[Pubmed:29729400]
Fitoterapia. 2018 Jul;128:36-42.
There is growing evidence that motherwort (Leonurus japonicus Houtt.), and Chinese patent medicines derived from motherwort, alleviate postpartum uterine subinvolution, as well as the effects on myocardial and cerebral ischemic injuries. We hypothesized that these beneficial effects of motherwort may be related to angiogenesis. To test this hypothesis, we investigated the angiogenic effects of motherwort total alkaloids and essential oil, as well as their respective primary components, on zebrafish embryos. Motherwort total alkaloids significantly increased angiogenesis in transgenic Tg (flk1: EGFP) zebrafish embryos treated with sunitinib, as did Stachydrine, the most abundant alkaloid produced by motherwort. Unexpectedly, motherwort essential oil was toxic to zebrafish embryos. Our results indicated, for the first time, that motherwort alkaloids were potent angiogenic agents, while even low concentrations of motherwort essential oil were toxic. As angiogenesis is a critical aspect of postpartum recovery, our results provide evidence for traditional application of motherwort water decoction and its Chinese patent medicines (e.g. motherwort injection) to promote postpartum recovery.
[Standard decoction of Leonuri Herba].[Pubmed:29218937]
Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(18):3523-3529.
To build an evaluation standard for quality of Leonuri Herba standard decoction. 13 batches of Leonuri Herba standard decoction with different quality were prepared. The contents of leonurine hydrochloride and Stachydrine hydrochloride were determined; then the transfer rate and the extract rate were calculated and pH value was measured; and HPLC fingerprint method was established for analysis. The results of 13 batches of samples revealed that the transfer rate of leonurine hydrochloride and Stachydrine hydrochloride was 30.0%-53.4% and 67.0%-82.6%, respectively; the extract rate was 12.1%-18.3% and the pH value was 5.87-6.22. Moreover, 12 common chromatographic peaks were determined based on fingerprint by using Similarity Evaluation System for Chromatographic fingerprint of Traditional Chinese Medicine (2012A). The similarity of 13 batches of samples was analyzed and compared, and the results showed that the similarity was higher than 0.9. In this study, the preparation method for Leonuri Herba decoction was standard, with high similarity in fingerprint, showing high precision, stability and repeatability in fingerprint analysis. Thus, this study can provide a reference for the quality control of Leonuri Herba dispensing granules.
Phytochemistry and pharmacology of the genus Leonurus: The herb to benefit the mothers and more.[Pubmed:29335190]
Phytochemistry. 2018 Mar;147:167-183.
Plants belonging to the genus Leonurus, also named motherwort, are traditionally used for anti-gynecological disorder in East Asia, and for sedative in Europe. Chemical investigation of the genus Leonurus not only enriched the natural products library, but also enlarged the pharmacological application of this traditional herb. In this review, we systematically summarized the structures of 259 compounds isolated from the genus Leonurus, featured with 147 labdane diterpenoids. The reported bioactivity studies up to 2017 are presented in the second part, with the main focus on the isolated compounds and also concerning the extracts. In addition to the traditional uterine contraction and sedative activity, recently the cardiovascular protection effect of leonurine has drawn most attention. Other than that, neuroprotection, anti-inflammation, anti-cancer, anti-platelet aggregation and many other activities have been assigned to various compounds from the genus Leonurus. Among 70 bioactivity references cited in this review, 57% of them were concentrated on two alkaloids (leonurine and Stachydrine), whereas only 20% are about the 147 diterpenoids. Anti-inflammation is the major bioactivity discovered so far for the labdane diterpenoids from the genus Leonurus, whose further therapeutic potential still remains for exploration.
[Optimize concentrate process of alkaloid from Leonurus japonicus by ultrafultration-nanofiltration coupling technology].[Pubmed:28945032]
Zhongguo Zhong Yao Za Zhi. 2017 Jan;42(1):100-106.
To optimize the concentrate process of alkaloid from Leonurus japonicus by nanofiltration-ultrafiltration coupling technology with response surface methodology. The experiment showed that after ultrafiltration pre-treatment, the total protein removal rate was 94.38% in aqueous extract from L. japonicus, and the nanofiltration technology had obvious advantages over the conventional concentrate process. The optimal concentrate conditions were as followsmolecular weight cut-off 450, pH 3.07, concentration of Stachydrine hydrochloride 80.15 mg*L(-)(1), and concentration of the total alkaloid 285.73 mg*L(-)(1). The cut-off rate was 93.37% and 95.85% respectively for Stachydrine hydrochloride and the total alkaloid under the optimum conditions, with a relative error of 0.79% and 1.16% respectively. The combination of Box-Behnken design and response surface analysis can well optimize the concentrate process of L. japonicus by nanofiltration, and the results provide the basis for nanofiltration concentrate for heat-sensitive traditional Chinese medicine.
Stachydrine ameliorates pressure overload-induced diastolic heart failure by suppressing myocardial fibrosis.[Pubmed:28979698]
Am J Transl Res. 2017 Sep 15;9(9):4250-4260. eCollection 2017.
Stachydrine (Sta), a major constituent of Leonurus japonicus Houtt, has been reported to possess numerous cardioprotective effects. In this study, we evaluated the effect of Sta on pressure overload-induced diastolic heart failure in rats and investigated the mechanisms underlying the effect. Wistar rats were randomized to transverse aortic constriction (TAC) or sham operation. After 3 days, the rats that underwent TAC were randomized to treatment for a total of four experimental groups (n=10 each group): sham operation, TAC only, TAC + telmisartan (Tel), and TAC + Stachydrine (Sta). After 12 weeks, we evaluated left ventricular hypertrophy, function, and fibrosis by echocardiography, pressure-volume loop analysis, and histology. In addition, levels of fibrosis-related proteins in the heart were determined by Western blot analysis. Our results showed that Sta significantly suppressed TAC-induced cardiac hypertrophy, and TAC-induced increases in heart weight/body weight and heart weight/tibial length. In addition, Sta attenuated TAC-induced decreases in left ventricular ejection fraction and improved other hemodynamic parameters. Compared with the TAC only group, rats treated with Sta exhibited significant decreases in interstitial and perivascular fibrosis, TGF-betaR1 protein levels, and phosphorylation of Smad2/3; however, protein levels of TGF-beta1, TGF-betaR2, and Smad4 did not differ significantly between the two groups. Taken together, our results demonstrate that Sta protects against diastolic heart failure by attenuating myocardial hypertrophy and fibrosis via the TGF-beta/Smad pathway.
Quality assessment of Herba Leonuri based on the analysis of multiple components using normal- and reversed-phase chromatographic methods.[Pubmed:28960719]
J Sep Sci. 2017 Dec;40(23):4482-4494.
A novel, improved, and comprehensive method for quality evaluation and discrimination of Herba Leonuri has been developed and validated based on normal- and reversed-phase chromatographic methods. To identify Herba Leonuri, normal- and reversed-phase high-performance thin-layer chromatography fingerprints were obtained by comparing the colors and Rf values of the bands, and reversed-phase high-performance liquid chromatography fingerprints were obtained by using an Agilent Poroshell 120 SB-C18 within 28 min. By similarity analysis and hierarchical clustering analysis, we show that there are similar chromatographic patterns in Herba Leonuri samples, but significant differences in counterfeits and variants. To quantify the bio-active components of Herba Leonuri, reversed-phase high-performance liquid chromatography was performed to analyze syringate, leonurine, quercetin-3-O-robiniaglycoside, hyperoside, rutin, isoquercitrin, wogonin, and genkwanin simultaneously by single standard to determine multi-components method with rutin as internal standard. Meanwhile, normal-phase high-performance liquid chromatography was performed by using an Agilent ZORBAX HILIC Plus within 6 min to determine trigonelline and Stachydrine using trigonelline as internal standard. Innovatively, among these compounds, bio-active components of quercetin-3-O-robiniaglycoside and trigonelline were first determined in Herba Leonuri. In general, the method integrating multi-chromatographic analyses offered an efficient way for the standardization and identification of Herba Leonuri.


