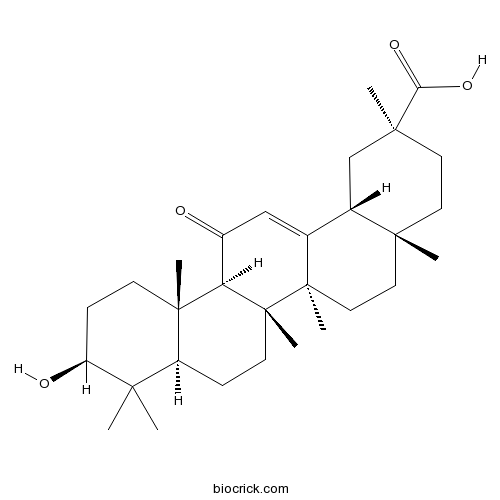
Glycyrrhetinic acidmajor component of licorice CAS# 471-53-4 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- PF-03814735
Catalog No.:BCC2184
CAS No.:942487-16-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 471-53-4 | SDF | Download SDF |
| PubChem ID | 10114 | Appearance | White powder |
| Formula | C30H46O4 | M.Wt | 470.64 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Enoxolone;18β-Glycyrrhetic acid; Glycyrrhetin; Subglycyrrhelinic acid; Uralenic acid | ||
| Solubility | DMSO : ≥ 250 mg/mL (531.15 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S,4aS,6aR,6aS,6bR,8aR,10S,12aS,14bR)-10-hydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-13-oxo-3,4,5,6,6a,7,8,8a,10,11,12,14b-dodecahydro-1H-picene-2-carboxylic acid | ||
| SMILES | CC1(C2CCC3(C(C2(CCC1O)C)C(=O)C=C4C3(CCC5(C4CC(CC5)(C)C(=O)O)C)C)C)C | ||
| Standard InChIKey | MPDGHEJMBKOTSU-YKLVYJNSSA-N | ||
| Standard InChI | InChI=1S/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21-,22-,23+,26+,27-,28-,29+,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glycyrrhetinic acid, an AChE activator, has anti-inflammatory,and antileukaemic activities. It is a potent inducer of mitochondrial permeability transition and can trigger the pro-apoptotic pathway, it has a low but definite affinity for mineralocorticoid receptors and thus appears to have a direct mineralocorticoid action. |
| Targets | PERK | CDK | E2F‑1 | AChE |
| In vitro | Glycyrrhetinic acid induces G1‑phase cell cycle arrest in human non‑small cell lung cancer cells through endoplasmic reticulum stress pathway.[Pubmed: 25573651]Int J Oncol. 2015 Mar;46(3):981-8.Glycyrrhetinic acid (GA) is a natural compound extracted from liquorice, which is often used in traditional Chinese medicine.
Glycyrrhetinic acid-induced permeability transition in rat liver mitochondria.[Pubmed: 14637195]Biochem Pharmacol. 2003 Dec 15;66(12):2375-9.Glycyrrhetinic acid, a hydrolysis product of one of the main constituents of licorice, the triterpene glycoside of glycyrrhizic acid, when added to rat liver mitochondria at micromolar concentrations induces swelling, loss of membrane potential, pyridine nucleotide oxidation, and release of cytochrome c and apoptosis inducing factor. These changes are Ca(2+) dependent and are prevented by cyclosporin A, bongkrekic acid, and N-ethylmaleimide. All these observations indicate that Glycyrrhetinic acid is a potent inducer of mitochondrial permeability transition and can trigger the pro-apoptotic pathway. |
| In vivo | The antiinflammatory activity of glycyrrhetinic acid and derivatives.[Reference: WebLink]J. Pharm. Pharmacol., 2011, 10(1):613-20.The anti-inflammatory activities of different fractions of Glycyrrhetinic acid or glycyrrhetic acid and some of its derivatives have been assessed in laboratory animals. Some, but not all, preparations have been found to be active using four established methods for testing anti-inflammatory drugs. The findings provide a scientific basis for the clinical use of these compounds in inflammatory diseases, and may explain the discrepancies in the early clinical trials with this drug. |
| Kinase Assay | Amino derivatives of glycyrrhetinic acid as potential inhibitors of cholinesterases.[Pubmed: 24853320]Bioorg Med Chem. 2014 Jul 1;22(13):3370-8.The development of remedies against the Alzheimer's disease (AD) is one of the biggest challenges in medicinal chemistry nowadays. Although not completely understood, there are several strategies fighting this disease or at least bringing some relief. During the progress of AD, the level of acetylcholine (ACh) decreases; hence, a therapy using inhibitors should be of some benefit to the patients. Drugs presently used for the treatment of AD inhibit the two ACh controlling enzymes, acetylcholinesterase as well as butyrylcholinesterase; hence, the design of selective inhibitors is called for.
|
| Cell Research | Glycyrrhetinic Acid Induces Apoptosis in Leukemic HL60 Cells Through Upregulating of CD95/ CD178.[Pubmed: 25635254]Int J Mol Cell Med. 2014 Fall;3(4):272-8.Acute leukemia is characterized by the accumulation of neoplastic cells in the bone marrow and peripheral blood. Currently, chemotherapy and differentiating agents have been used for the treatment of leukemia. Recently, plant extracts, either alone or in combination with chemo agents, have been proposed to be used for the treatment of cancers.
|

Glycyrrhetinic acid Dilution Calculator

Glycyrrhetinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1248 mL | 10.6238 mL | 21.2477 mL | 42.4953 mL | 53.1192 mL |
| 5 mM | 0.425 mL | 2.1248 mL | 4.2495 mL | 8.4991 mL | 10.6238 mL |
| 10 mM | 0.2125 mL | 1.0624 mL | 2.1248 mL | 4.2495 mL | 5.3119 mL |
| 50 mM | 0.0425 mL | 0.2125 mL | 0.425 mL | 0.8499 mL | 1.0624 mL |
| 100 mM | 0.0212 mL | 0.1062 mL | 0.2125 mL | 0.425 mL | 0.5312 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Glycyrrhetinic acid (enoxolone) is a major component of a plant called licorice. It has been found to have both antiviral and antifungal activities. It is able to carboxilate the replication of DNA and inhibits the production of microbial toxins and enzymes [1] [2]. In rats, 18β-Glycyrrhetinic acid potently inhibited 11β-hydroxysteroid dehydrogenase (11β-HSD) activity of hepatic and renal homogenates with IC50 values of 0.09 μm and 0.36 μm, respectively [3].
Before glucocorticoids bind the mineralocorticoid receptor (MR), the selectivity of MR for aldosterone can be exerted by enzymes, and hence competing glucocorticoids is inactivated. 11β-HSD-1 and 11β-HSD-2 and 11β-HSD-3 are three of the enzymes. 11β-HSD-1 has bidirectional activity and a Km value in the micromolar range. It is NADP+-dependent. 11β-HSD-2 exhibits only oxidase activity and has a Km in the nanomolar range. It is NAD+-dependent. In the kidney, it colocalizes with the MR [4].
JEG-3 cell line was derived from a human choriocarcinoma. In this cell line, the enzyme activity of 11β-HSD-3 was inhibited by glycyrrhetinic acid [4].
In rats, 3 h after the administration of glycyrrhetinic acid at concentration of 200 mg/kg, p.o. significantly inhibited 11β-HSD activity in the kidney and the liver. In addition, glycyrrhetinic acid slightly increased the circulating corticosterone level. In differential inhibitory effects on 11β-HSD isozyme activity, the 11-, 24- and 30-positions of glycyrrhetinic acid may play important roles. This had been showed by Data [3].
References:
[1]. Salari MH, Sohrabi N, Kadkhoda Z, et al. Antibacterial Effects of Enoxolone on Periodontopathogenic and Capnophilic Bacteria Isolated from Specimens of Periodontitis Patients. Iranian Biomedical Journal, 2003, 7(1):39-42.
[2]. Bahmani M, Rafieian-Kopaei M, Jeloudari M, et al. A review of the health effects and uses of drugs of plant licorice (Glycyrrhiza glabra L.) in Iran. Asian Pacific Journal of Tropical Disease, 2015, 5: 127-29.
[3]. Shimoyama Y, Hirabayashi K, Matsumoto H, et al. Effects of glycyrrhetinic acid derivatives on hepatic and renal 11beta-hydroxysteroid dehydrogenase activities in rats. J Pharm Pharmacol, 2003, 55(6):811-7.
[4]. Gomez-Sanchez EP, Cox D, Foecking M, et al. 11 beta-hydroxysteroid dehydrogenases of the choriocarcinoma cell line JEG-3 and their inhibition by glycyrrhetinic acid and other natural substances. Steroids, 1996, 61(3):110-5.
- 8-Amino-7-oxononanoic acid
Catalog No.:BCN1778
CAS No.:4707-58-8
- Atraric acid
Catalog No.:BCN5521
CAS No.:4707-47-5
- alpha-Lapachone
Catalog No.:BCN5520
CAS No.:4707-33-9
- Beta-Lapachone
Catalog No.:BCC5088
CAS No.:4707-32-8
- Benzoyl-DL-methionine
Catalog No.:BCC8863
CAS No.:4703-38-2
- Cineole
Catalog No.:BCN2686
CAS No.:470-82-6
- 1-Kestose
Catalog No.:BCN8292
CAS No.:470-69-9
- Stachyose tetrahydrate
Catalog No.:BCC8252
CAS No.:470-55-3
- Marinobufagin
Catalog No.:BCC9238
CAS No.:470-42-8
- Cinobufagin
Catalog No.:BCN5367
CAS No.:470-37-1
- Isoalantolactone
Catalog No.:BCN4955
CAS No.:470-17-7
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Isocolumbin
Catalog No.:BCN5361
CAS No.:471-54-5
- alpha-Boswellic acid
Catalog No.:BCN5522
CAS No.:471-66-9
- Dipterocarpol
Catalog No.:BCN5523
CAS No.:471-69-2
- (-)-Steviol
Catalog No.:BCN8358
CAS No.:471-80-7
- Stachydrine
Catalog No.:BCN8384
CAS No.:471-87-4
- Bufotaline
Catalog No.:BCN5368
CAS No.:471-95-4
- (E)-Aldosecologanin
Catalog No.:BCN4631
CAS No.:471271-55-3
- Boc-D-Ser(Bzl)-OH
Catalog No.:BCC3448
CAS No.:47173-80-8
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- Ruscogenin
Catalog No.:BCN6287
CAS No.:472-11-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Telocinobufagin
Catalog No.:BCN2359
CAS No.:472-26-4
Amino derivatives of glycyrrhetinic acid as potential inhibitors of cholinesterases.[Pubmed:24853320]
Bioorg Med Chem. 2014 Jul 1;22(13):3370-8.
The development of remedies against the Alzheimer's disease (AD) is one of the biggest challenges in medicinal chemistry nowadays. Although not completely understood, there are several strategies fighting this disease or at least bringing some relief. During the progress of AD, the level of acetylcholine (ACh) decreases; hence, a therapy using inhibitors should be of some benefit to the patients. Drugs presently used for the treatment of AD inhibit the two ACh controlling enzymes, acetylcholinesterase as well as butyrylcholinesterase; hence, the design of selective inhibitors is called for. Glycyrrhetinic acid seems to be an interesting starting point for the development of selective inhibitors. Although its glycon, Glycyrrhetinic acid is known for being an AChE activator, several derivatives, altered in position C-3 and C-30, exhibited remarkable inhibition constants in micro-molar range. Furthermore, five representative compounds were subjected to three more enzyme assays (on carbonic anhydrase II, papain and the lipase from Candida antarctica) to gain information about the selectivity of the compounds in comparison to other enzymes. In addition, photometric sulforhodamine B assays using murine embryonic fibroblasts (NiH 3T3) were performed to study the cytotoxicity of these compounds. Two derivatives, bearing either a 1,3-diaminopropyl or a 1H-benzotriazolyl residue, showed a BChE selective inhibition in the single-digit micro-molar range without being cytotoxic up to 30muM. In silico molecular docking studies on the active sites of AChE and BChE were performed to gain a molecular insight into the mode of action of these compounds and to explain the pronounced selectivity for BChE.
Glycyrrhetinic acid-induced permeability transition in rat liver mitochondria.[Pubmed:14637195]
Biochem Pharmacol. 2003 Dec 15;66(12):2375-9.
Glycyrrhetinic acid, a hydrolysis product of one of the main constituents of licorice, the triterpene glycoside of glycyrrhizic acid, when added to rat liver mitochondria at micromolar concentrations induces swelling, loss of membrane potential, pyridine nucleotide oxidation, and release of cytochrome c and apoptosis inducing factor. These changes are Ca(2+) dependent and are prevented by cyclosporin A, bongkrekic acid, and N-ethylmaleimide. All these observations indicate that Glycyrrhetinic acid is a potent inducer of mitochondrial permeability transition and can trigger the pro-apoptotic pathway.
Glycyrrhetinic Acid Induces Apoptosis in Leukemic HL60 Cells Through Upregulating of CD95/ CD178.[Pubmed:25635254]
Int J Mol Cell Med. 2014 Fall;3(4):272-8.
Acute leukemia is characterized by the accumulation of neoplastic cells in the bone marrow and peripheral blood. Currently, chemotherapy and differentiating agents have been used for the treatment of leukemia. Recently, plant extracts, either alone or in combination with chemo agents, have been proposed to be used for the treatment of cancers. The aim of the present research was to study the cytotoxicity and apoptosis effects of an active licorice-derived compound, Glycyrrhetinic acid (GA), on human leukemic HL60 cells. HL60 cells were cultured in RPMI1640 containing 10% fetal bovine serum. Cells were treated with different doses of GA and their viability and proliferation were detected by dye exclusion and 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assays. Apoptosis induction and expression of CD95 and CD178 were analyzed by flow cytometry. We observed that GA decreases cell viability and suppresses cells proliferation in a dose- dependent manner. In addition, our flow cytometry data show that GA not only induces apoptosis in HL60 cells, but also upregulates both CD95 and CD178 expression on the cell surface of these cells in a dose-dependent manner. The combination of GA with cytotoxic drugs and differentiation agents requires further investigation.
Glycyrrhetinic acid induces G1phase cell cycle arrest in human nonsmall cell lung cancer cells through endoplasmic reticulum stress pathway.[Pubmed:25573651]
Int J Oncol. 2015 Mar;46(3):981-8.
Glycyrrhetinic acid (GA) is a natural compound extracted from liquorice, which is often used in traditional Chinese medicine. The purpose of the present study was to investigate the antitumor effect of GA in human nonsmall cell lung cancer (NSCLC), and its underlying mechanisms in vitro. We have shown that GA suppressed the proliferation of A549 and NCIH460 cells. Flow cytometric analysis showed that GA arrested cell cycle in G0/G1 phase without inducing apoptosis. Western blot analysis indicated that GA mediated G1phase cell cycle arrest by upregulation of cyclindependent kinase inhibitors (CKIs) (p18, p16, p27 and p21) and inhibition of cyclins (cyclinD1, D3 and E) and cyclindependent kinases (CDKs) (CDK4, 6 and 2). GA also maintained pRb phosphorylation status, and inhibited E2F transcription factor 1 (E2F1) in both cell lines. GA upregulated the unfolded proteins, Bip, PERK and ERP72. Accumulation of unfolded proteins in the endoplasmic reticulum (ER) triggered the unfolded protein response (UPR), which could be the mechanism by which GA inhibited cell proliferation in NSCLC cells. GA then coordinated the induction of ER chaperones, which decreased protein synthesis and induced cell cycle arrest in the G1 phase. This study provides experimental evidence to support the development of GA as a chemotherapeutic agent for NSCLC.
Glycyrrhetinic Acid inhibits cell growth and induces apoptosis in ovarian cancer a2780 cells.[Pubmed:25364659]
Adv Pharm Bull. 2014 Oct;4(Suppl 1):437-41.
PURPOSE: Accumulating evidence indicates that glycyrrhizin (GZ) and its hydrolyzed metabolite 18-beta Glycyrrhetinic acid (GA) exhibit anti-inflammatory and anticancer activities. The objective of this study was to examine the in vitro cytotoxic activity of GA on human ovarian cancer A2780 cells. METHODS: A2780 cells were cultured in RPMI1640 containing 10% fetal bovine serum. Cells were treated with different doses of GA and cell viability and proliferation were detected by dye exclusion and 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assays. Apoptosis induction and expression of Fas and Fas ligand (FasL) were analyzed by flow cytometry. RESULTS: We observed that GA decreases cell viability and suppressed cells proliferation in a dose-dependent manner as detected by dye-exclusion and XTT assayes. In addition, our flow cytometry data show that GA not only induces apoptosis in A2780 cells but also upregulates both Fas and FasL on these cells in a dose-dependent manner. CONCLUSION: we demonstrate that GA causes cell death in A2780 cells by inducing apoptosis.


