CinobufaginCAS# 470-37-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 470-37-1 | SDF | Download SDF |
| PubChem ID | 11969542 | Appearance | White powder |
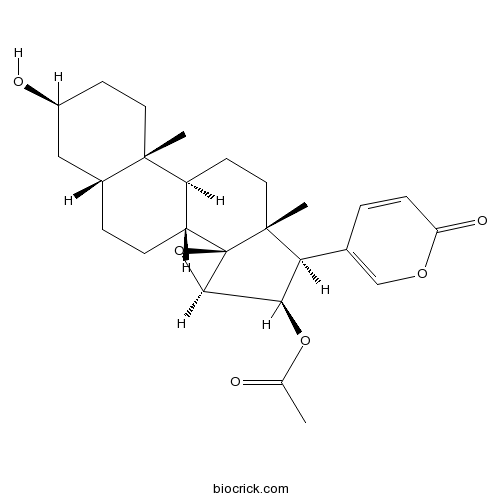
| Formula | C26H34O6 | M.Wt | 442.55 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | Cinobufagine | ||
| Solubility | DMSO : ≥ 43 mg/mL (97.17 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(1R,2S,4R,5R,6R,7R,10S,11S,14S,16R)-14-hydroxy-7,11-dimethyl-6-(6-oxopyran-3-yl)-3-oxapentacyclo[8.8.0.02,4.02,7.011,16]octadecan-5-yl] acetate | ||
| SMILES | CC(=O)OC1C(C2(CCC3C(C24C1O4)CCC5C3(CCC(C5)O)C)C)C6=COC(=O)C=C6 | ||
| Standard InChIKey | SCULJPGYOQQXTK-OLRINKBESA-N | ||
| Standard InChI | InChI=1S/C26H34O6/c1-14(27)31-22-21(15-4-7-20(29)30-13-15)25(3)11-9-18-19(26(25)23(22)32-26)6-5-16-12-17(28)8-10-24(16,18)2/h4,7,13,16-19,21-23,28H,5-6,8-12H2,1-3H3/t16-,17+,18+,19-,21+,22-,23-,24+,25-,26-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cinobufagin, a kind of Chinese materia medica with antitumor effect, is widely used in clinical practice, especially in anti-liver cancer, it also has anti-hepatitis B virus activity . Cinobufagin inhibits the proliferation and induces apoptosis, may be related to the mitochondria-mediated pathway and GSK-3β/NF-κB pathway. Cinobufagin can significantly relieve cancer pain in mice and raised their pain threshold, mainly upregulating the expression levels of β -END and μ -OR in the hind paw tumor and adjacent tissue.Cinobufagin and bufalin exhibit cardiotonic and natriuretic activities, they also have inhibitory effects on steroidogenesis of aldosterone and cortisol. |
| Targets | ERK | JNK | p38MAPK | Caspase | PARP | ROS | GSK-3 | NF-kB | p65 | HBV |
| In vitro | Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway.[Pubmed: 25982794]Immunopharmacol Immunotoxicol. 2015 May 18:1-9.Cinobufagin (CBG) is a cardiotoxic bufanolide steroid secreted by the skin and parotid venom glands of the Asiatic toad Bufo bufo gargarizans (called Chan-Su). Although CBG is known to exhibit anti-cancer activities, very little is known about its potential mechanism(s) of action. In this study, we investigated whether CBG mediates its effect through the modulation of the mitogen-activated protein kinases (MAPKs) signaling pathway in human multiple myeloma (MM) U266 cells. Anti-hepatitis B virus activities of cinobufacini and its active components bufalin and cinobufagin in HepG2.2.15 cells.[Pubmed: 20930383]Biol Pharm Bull. 2010;33(10):1728-32.Cinobufacini (Huachansu) is a Chinese medicine prepared from the skin of Bufo bufo gargarizans Cantor (Bufonidae), which has long been used in traditional Chinese medicine (TCM). The aim of present study was to examine the anti-hepatitis B virus (HBV) activities of cinobufacini and its active components bufalin and Cinobufagin in the human HBV-transfected cell line HepG2.2.15.
|
| In vivo | A Study on the Mechanism of Cinobufagin in the Treatment of Paw Cancer Pain by Modulating Local β -Endorphin Expression In Vivo.[Pubmed: 24187573]Evid Based Complement Alternat Med. 2013;2013:851256.Cinobufagin has been widely used in the treatment of carcinoma and plays an important role in the relief of cancer pain. But the involved mechanism remains unknown.
To investigate the changes in thermal and mechanical hyperalgesia in paw cancer pain in mice and the action mechanism of Cinobufagin using a paw cancer pain model.
|
| Kinase Assay | Resibufogenin and cinobufagin activate central neurons through an ouabain-like action.[Pubmed: 25420080]PLoS One. 2014 Nov 24;9(11):e113272.Cinobufagin and resibufogenin are two major effective bufadienolides of Chan su (toad venom), which is a Chinese medicine obtained from the skin venom gland of toads and is used as a cardiotonic and central nervous system (CNS) respiratory agent, an analgesic and anesthetic, and as a remedy for ulcers. Many clinical cases showed that Chan su has severe side-effects on the CNS, causing shortness of breath, breathlessness, seizure, coma and cardiac arrhythmia.
|
| Cell Research | The glycogen synthase kinase-3β/nuclear factor-kappa B pathway is involved in cinobufagin-induced apoptosis in cultured osteosarcoma cells.[Pubmed: 23164673]Effects of cinobufagin on apoptosis in U-2OS osteosarcomas cells.[Pubmed: 24844018]Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014 Mar;28(3):349-53.To investigate the effects of Cinobufagin on the apoptosis in U-2OS osteosarcomas cells (U-2OS cells) and explore its potential mechanism.
Toxicol Lett. 2013 Apr 12;218(2):129-36.Cinobufagin, a major component of cinobufacini (huachansu), is an important cardenolidal steroid. Several studies have suggested that Cinobufagin has potent anti-cancer effects. The present study examines the apoptosis-inducing activity and the underlying mechanism of action of Cinobufagin in osteosarcoma (OS) cells.
|

Cinobufagin Dilution Calculator

Cinobufagin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2596 mL | 11.2982 mL | 22.5963 mL | 45.1926 mL | 56.4908 mL |
| 5 mM | 0.4519 mL | 2.2596 mL | 4.5193 mL | 9.0385 mL | 11.2982 mL |
| 10 mM | 0.226 mL | 1.1298 mL | 2.2596 mL | 4.5193 mL | 5.6491 mL |
| 50 mM | 0.0452 mL | 0.226 mL | 0.4519 mL | 0.9039 mL | 1.1298 mL |
| 100 mM | 0.0226 mL | 0.113 mL | 0.226 mL | 0.4519 mL | 0.5649 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cinobufagin, a kind of Chinese materia medica with antitumor effect, is widely used in clinical practice, especially in anti-liver cancer. IC50 value: Target: In vitro: Cinobufagin inhibited proliferation of cancer cells at doses of 0.1, 1, or 10 μM after 2–4 days of culture. Cytotoxicity of cinobufagin on the DU145 and LNCaP cells was dose-dependent. Cinobufagin increased [Ca2+]i and apoptosis in cancer cells after a 24-hr culture as well as caspase 3 activities in DU145 and PC3 cells and caspase 9 activities in LNCaP cells [1]. Cinobufagin suppresses cell proliferation and causees apoptosis in prostate cancer cells via a sequence of apoptotic modulators, including Bax, cytochrome c and caspases [2]. In vivo:
References:
[1]. Zhang G, et al. Cinobufagin inhibits tumor growth by inducing intrinsic apoptosis through AKT signaling pathway in human nonsmall cell lung cancer cells. Oncotarget. 2016 Mar 3.
[2]. Yu Y, et al. Immunomodulatory Effects of Cinobufagin on Murine Lymphocytes and Macrophages. Evid Based Complement Alternat Med. 2015;2015:835263.
[3]. Baek SH, et al. Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway. Immunopharmacol Immunotoxicol. 2015 Jun;37(3):265-73.
[4]. Jiun-Yih Yeh, et al. Effects of bufalin and cinobufagin on the proliferation of androgen dependent and independent prostate cancer cells. The Prostate
Volume 54, Issue 2, pages 112–124, 1 February 2003
[5]. Yu CH, et al. Apoptotic signaling in bufalin- and cinobufagin-treated androgen-dependent and -independent human prostate cancer cells. Cancer Science, 2008, 99 (12): 2467-2476
[6]. Peng Bei, et al. Progress in study on basic and clinical application in treating liver cancer by Cinobufagin Injection. Drug Evaluation Research, 2011-01
- Isoalantolactone
Catalog No.:BCN4955
CAS No.:470-17-7
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Carbenicillin
Catalog No.:BCC5192
CAS No.:4697-36-3
- 5'-IMPdisodium salt
Catalog No.:BCN8175
CAS No.:4691-65-0
- Jervine
Catalog No.:BCN2975
CAS No.:469-59-0
- Cycloeucalenol
Catalog No.:BCN5519
CAS No.:469-39-6
- Hamamelitannin
Catalog No.:BCC8182
CAS No.:469-32-9
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- Cimilactone A
Catalog No.:BCN7948
CAS No.:468733-06-4
- 3-Benzofurancarboxaldehyde
Catalog No.:BCC8622
CAS No.:4687-25-6
- Dihydrocorynantheine
Catalog No.:BCN3747
CAS No.:4684-43-9
- Picrinine
Catalog No.:BCN5518
CAS No.:4684-32-6
- Marinobufagin
Catalog No.:BCC9238
CAS No.:470-42-8
- Stachyose tetrahydrate
Catalog No.:BCC8252
CAS No.:470-55-3
- 1-Kestose
Catalog No.:BCN8292
CAS No.:470-69-9
- Cineole
Catalog No.:BCN2686
CAS No.:470-82-6
- Benzoyl-DL-methionine
Catalog No.:BCC8863
CAS No.:4703-38-2
- Beta-Lapachone
Catalog No.:BCC5088
CAS No.:4707-32-8
- alpha-Lapachone
Catalog No.:BCN5520
CAS No.:4707-33-9
- Atraric acid
Catalog No.:BCN5521
CAS No.:4707-47-5
- 8-Amino-7-oxononanoic acid
Catalog No.:BCN1778
CAS No.:4707-58-8
- Glycyrrhetinic acid
Catalog No.:BCN5942
CAS No.:471-53-4
- Isocolumbin
Catalog No.:BCN5361
CAS No.:471-54-5
- alpha-Boswellic acid
Catalog No.:BCN5522
CAS No.:471-66-9
Resibufogenin and cinobufagin activate central neurons through an ouabain-like action.[Pubmed:25420080]
PLoS One. 2014 Nov 24;9(11):e113272.
Cinobufagin and resibufogenin are two major effective bufadienolides of Chan su (toad venom), which is a Chinese medicine obtained from the skin venom gland of toads and is used as a cardiotonic and central nervous system (CNS) respiratory agent, an analgesic and anesthetic, and as a remedy for ulcers. Many clinical cases showed that Chan su has severe side-effects on the CNS, causing shortness of breath, breathlessness, seizure, coma and cardiac arrhythmia. We used whole-cell recordings from brain slices to determine the effects of bufadienolides on excitability of a principal neuron in main olfactory bulb (MOB), mitral cells (MCs), and the cellular mechanism underlying the excitation. At higher concentrations, Cinobufagin and resibufogenin induced irreversible over-excitation of MCs indicating a toxic effect. At lower concentrations, they concentration-dependently increased spontaneous firing rate, depolarized the membrane potential of MCs, and elicited inward currents. The excitatory effects were due to a direct action on MCs rather than an indirect phasic action. Bufadienolides and ouabain had similar effects on firing of MCs which suggested that bufadienolides activated neuron through a ouabain-like effect, most likely by inhibiting Na+/K+-ATPase. The direct action of bufadienolide on brain Na+ channels was tested by recordings from stably Nav1.2-transfected cells. Bufadienolides failed to make significant changes of the main properties of Nav1.2 channels in current amplitude, current-voltage (I-V) relationships, activation and inactivation. Our results suggest that inhibition of Na+/K+-ATPase may be involved in both the pharmacological and toxic effects of bufadienolide-evoked CNS excitation.
The glycogen synthase kinase-3beta/nuclear factor-kappa B pathway is involved in cinobufagin-induced apoptosis in cultured osteosarcoma cells.[Pubmed:23164673]
Toxicol Lett. 2013 Apr 12;218(2):129-36.
Cinobufagin, a major component of cinobufacini (huachansu), is an important cardenolidal steroid. Several studies have suggested that Cinobufagin has potent anti-cancer effects. The present study examines the apoptosis-inducing activity and the underlying mechanism of action of Cinobufagin in osteosarcoma (OS) cells. Our results showed that Cinobufagin potently inhibited the proliferation of U2OS, MG63 and SaOS-2 cells. Significant increases in G2/M cell-cycle arrest and apoptosis in OS cells were also observed. The expression levels of several apoptotic proteins were assessed after Cinobufagin treatment in U2OS cells. Among them, xIAP, cIAP-1, survivin and Bcl-2 levels decreased remarkably, while the levels of Bax and cleaved-PARP increased. Furthermore, we validated the inhibition of GSK-3beta/NF-kappaB signaling following Cinobufagin treatment. Western blots showed a decrease in nuclear p65 protein expression after exposure to different concentrations of Cinobufagin, while the phosphorylation of GSK-3beta was simultaneously increased. Transduction with constitutively active forms of GSK-3beta could protect against the downregulation of p65 and upregulation of cleaved-PARP that are induced by Cinobufagin treatment. However, combined treatment with Cinobufagin and SB216367 resulted in a significant reduction in p65 and an increase in cleaved-PARP in U2OS cells. Altogether, these results show that Cinobufagin is a promising agent for the treatment of OS. These studies are the first to reveal the involvement of the GSK-3beta/NF-kappaB pathway in Cinobufagin-induced apoptosis.
[Effects of cinobufagin on apoptosis in U-2OS osteosarcomas cells].[Pubmed:24844018]
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014 Mar;28(3):349-53.
OBJECTIVE: To investigate the effects of Cinobufagin on the apoptosis in U-2OS osteosarcomas cells (U-2OS cells) and explore its potential mechanism. METHODS: The cytostatic effects of Cinobufagin (10, 20, 50, 100, 200, and 400 nmol/L) on U-2OS cells were evaluated by MTT assay at 24, 48, and 72 hours after culture; simple U-2OS cells served as control group. The impact of Cinobufagin (100 nmol/L) on the apoptosis in U-2OS cells was determined by flow cytometry at 48 hours after culture, which were treated with Cinobufagin (experimental group) or with Cinobufagin plus Z-VAD-FMK (control group), and simple U-2OS cells served as blank control group. The Caspase-3 activity was measured by Caspase-3 activity assay kit at 48 hours after culture, which were treated with Cinobufagin (20, 50, and 100 nmol/L), and simple U-2OS cells served as control group. The expression of apoptosis signal pathway related proteins in U-2OS cells treated with Cinobufagin were detected by Western blot at 48 hours after culture, which were treated with Cinobufagin (20, 50, and 100 nmol/L), and simple U-2OS cells served as control group. RESULTS: The results of MTT assay showed that Cinobufagin inhibited the proliferation of U-2OS cells in a dose- and time-dependent manners. At each time point, the growth rate of U-2OS cells was significantly reduced with the increasing Cinobufagin concentration, and as time prolonged, the growth rate of U-2OS cells behaved the same way in the same group. There were significant differences among different time points and groups (P < 0.05). The apoptotic rate of experimental group (46.87% +/- 11.23%) was significantly higher than that of the control group (2.34% +/- 0.98%) and blank control group (1.04% +/- 0.25%) (P < 0.05). The Caspase-3 activity in 20, 50, and 100 nmol/L groups were 1.14 +/- 0.32, 1.31 +/- 0.41, and 1.92 +/- 0.54, respectively, which were significantly higher than that in control group (P < 0.05). Compared with 20and 50 nmol/L groups, 100 nmol/L group significantly increased the Caspase-3 activity in U-2OS cells (P < 0.05). Compared with the control group, the expressions of cleaved Caspase-3, cleaved Caspase-9, and Bax were obviously up-regulated; the Bcl-2 expression was down-regulated; and the ratio of Bax/Bcl-2 was increased in different Cinobufagin-treated groups (P < 0.05). The same tendency was seen in different Cinobufagin-treated goups, showing significant differences among groups (P < 0.05). CONCLUSION: Cinobufagin can inhibite the proliferation of U-2OS cells, and induce cell apoptosis. The potential mechanism of Cinobufagin-induced apoptosis may be related to the mitochondria-mediated pathway.
Anti-hepatitis B virus activities of cinobufacini and its active components bufalin and cinobufagin in HepG2.2.15 cells.[Pubmed:20930383]
Biol Pharm Bull. 2010;33(10):1728-32.
Cinobufacini (Huachansu) is a Chinese medicine prepared from the skin of Bufo bufo gargarizans Cantor (Bufonidae), which has long been used in traditional Chinese medicine (TCM). The aim of present study was to examine the anti-hepatitis B virus (HBV) activities of cinobufacini and its active components bufalin and Cinobufagin in the human HBV-transfected cell line HepG2.2.15. The hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and hepatitis B core-related antigen (HBcrAg) concentrations in cell culture medium were determined by chemiluminescent enzyme immunoassay after HepG2.2.15 cells were respectively treated with different concentrations of cinobufacini, bufalin, and Cinobufagin for 3 or 6 d. HBV DNA and mRNA were determined using transcription-mediated amplification and real-time polymerase chain reaction (PCR), respectively. On d 3, cinobufacini at a concentration of 1 microg/ml had no activity against HBV virological markers. However, on d 6, cinobufacini at 1 microg/ml effectively inhibited the secretion of HBsAg, HBeAg, and HBcrAg by 29.58, 32.87, and 42.52%. It was more potent than the positive control lamivudine (100 microg/ml). Bufalin and Cinobufagin slightly inhibited HBV antigen secretion. Treatment with cinobufacini, bufalin, or Cinobufagin had no anti-HBV effect on DNA in cell culture medium. Consistent with the HBV antigen reduction, HBV mRNA expression was markedly inhibited in comparison to the control when HepG2.2.15 cells were treated with cinobufacini, bufalin, or Cinobufagin. Results suggested that cinobufacini had more potent activity against HBV antigen secretion than its components bufalin and Cinobufagin and this inhibitory role was attributed to the specific inhibition of HBV mRNA expression.
A Study on the Mechanism of Cinobufagin in the Treatment of Paw Cancer Pain by Modulating Local beta -Endorphin Expression In Vivo.[Pubmed:24187573]
Evid Based Complement Alternat Med. 2013;2013:851256.
Background. Cinobufagin has been widely used in the treatment of carcinoma and plays an important role in the relief of cancer pain. But the involved mechanism remains unknown. Aim. To investigate the changes in thermal and mechanical hyperalgesia in paw cancer pain in mice and the action mechanism of Cinobufagin using a paw cancer pain model. Methods. 60 female mice were randomly divided into 5 groups: control group, model group, Cinobufagin group, Cinobufagin +NAL-M group, and morphine group; except ones in control group, mice were inoculated with H22 hepatoma cells in the right hind paw. From the 9th day after inoculation, mice were administrated drug once daily lasting for 8 days. The pain behavior was determined on the 2nd, 4th, 6th, and 8th days before and after administration. On the last day, they were sacrificed. The levels of beta -END, CRF, and IL-1 beta were analyzed by ELISA; immunohistochemistry was performed to detect the expressions of beta -END, POMC, and mu -OR in the tumor and adjacent tissue. Results. The thresholds of thermal pain and mechanical pain were significantly increased by Cinobufagin. Moreover, the expressions of beta -END, CRF, POMC, and mu -OR were significantly upregulated by Cinobufagin. The analgesic effect of Cinobufagin was blocked by the peripheral opioid receptor antagonist NAL-M. Conclusions. Cinobufagin significantly relieved cancer pain in mice and raised their pain threshold, mainly upregulating the expression levels of beta -END and mu -OR in the hind paw tumor and adjacent tissue.
Inhibitory effect of bufalin and cinobufagin on steroidogenesis via the activation of ERK in human adrenocortical cells.[Pubmed:21913902]
Br J Pharmacol. 2012 Mar;165(6):1868-1876.
BACKGROUND AND PURPOSE: Bufalin and Cinobufagin exhibit cardiotonic and natriuretic activities. The aim of this study was to evaluate the effects of bufalin and Cinobufagin on aldosterone and cortisol secretion and their mechanisms of action in human adrenocortical cells (NCI-H295). EXPERIMENTAL APPROACH: H295 cells were incubated with bufalin or Cinobufagin in the presence or absence of angiotensin II (Ang II), forskolin, 8-Br-cAMP, corticosterone or deoxycortisol. The role of ERK1/2 was studied by use of the inhibitor of MEK (U0126). The binding of transcription factor steroidogenic factor 1 (SF-1) to steroidogenic acute regulatory (StAR) gene promoter was analysed by EMSA. KEY RESULTS: Bufalin and Cinobufagin markedly inhibited basal, Ang II-, forskolin- or 8-Br-cAMP-stimulated aldosterone and cortisol secretion, and the conversions of corticosterone to aldosterone and deoxycortisol to cortisol. Bufalin and Cinobufagin also inhibited StAR protein expression and SF-1 binding to StAR gene promoter. They both increased phosphorylation of ERK1/2, and U0126 fully abolished these effects on ERK1/2 in H295 cells. Furthermore, U0126 reversed the inhibitory effects of bufalin and Cinobufagin on StAR protein expression and the binding of SF-1 to StAR gene promoter. However, U0126 did not completely reverse their inhibitory effects on aldosterone and cortisol release. CONCLUSIONS AND IMPLICATIONS: The inhibitory effects of bufalin and Cinobufagin on steroidogenesis of aldosterone and cortisol were associated with inhibition of aldosterone synthase and 11beta-hydroxylase, as well as the suppression of StAR protein expression and SF-1 binding to StAR promoter via the phosphorylation of ERK1/2 in H295 cells.
Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway.[Pubmed:25982794]
Immunopharmacol Immunotoxicol. 2015 Jun;37(3):265-73.
Cinobufagin (CBG) is a cardiotoxic bufanolide steroid secreted by the skin and parotid venom glands of the Asiatic toad Bufo bufo gargarizans (called Chan-Su). Although CBG is known to exhibit anti-cancer activities, very little is known about its potential mechanism(s) of action. In this study, we investigated whether CBG mediates its effect through the modulation of the mitogen-activated protein kinases (MAPKs) signaling pathway in human multiple myeloma (MM) U266 cells. We found that CBG caused the significant activation of ERK, JNK and p38 MAPK in U266 cells. CBG showed much higher cytotoxicity against U266 cells as compared to peripheral blood mononuclear cells (PBMC). Induction of CBG increased reactive oxygen species (ROS) generation from mitochondria, which is associated with the induction of apoptosis as characterized by increased sub-G1 DNA contents of cell cycle, positive Annexin V binding, activation of caspase-3 and cleavage of PARP. Inhibition of ROS generation by N-acetyl-l-cysteine (NAC) significantly prevented CBG-induced ERK, JNK and p38 MAPK activation and apoptosis. CBG also down-regulated the expression of various downstream gene products that mediate cell proliferation, survival, angiogenesis and metastasis. Interestingly, ERK, JNK and p38MAPK pharmacological inhibitors blocked CBG-induced MAPKs activation and ERK inhibitor (PD98059) also prevented the CBG-induced caspase-3 activation and PARP cleavage in U266 cells. Taken together, these findings suggest that CBG can act as a potent anticancer agent against MM and possibly exerts its effects through the ROS-mediated activation of ERK, JNK and p38 MAPK leading to the activation of caspase-3 in U266 cells.


