JervineHedgehog signaling Inhibitor CAS# 469-59-0 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

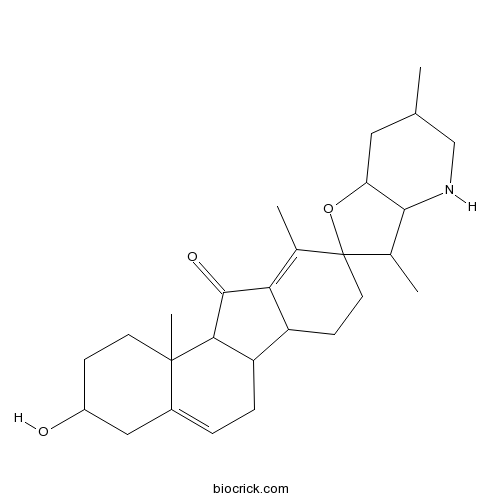
| Cas No. | 469-59-0 | SDF | Download SDF |
| PubChem ID | 222153 | Appearance | White powder |
| Formula | C27H39NO3 | M.Wt | 425.6 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | 11-Ketocyclopamine | ||
| Solubility | DMSO : 1 mg/mL (2.35 mM; Need ultrasonic) | ||
| Chemical Name | 3-hydroxy-3',6',10,11b-tetramethylspiro[1,2,3,4,6,6a,6b,7,8,11a-decahydrobenzo[a]fluorene-9,2'-3a,4,5,6,7,7a-hexahydro-3H-furo[3,2-b]pyridine]-11-one | ||
| SMILES | CC1CC2C(C(C3(O2)CCC4C5CC=C6CC(CCC6(C5C(=O)C4=C3C)C)O)C)NC1 | ||
| Standard InChIKey | CLEXYFLHGFJONT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H39NO3/c1-14-11-21-24(28-13-14)16(3)27(31-21)10-8-19-20-6-5-17-12-18(29)7-9-26(17,4)23(20)25(30)22(19)15(27)2/h5,14,16,18-21,23-24,28-29H,6-13H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Jervine exhibits cytotoxic activity against human tumor cell lines A549, PANC-1, SW1990 and NCI-H249, it induces COX-2 overexpression in human erythroleukemia cells. Jervine exhibits potent toxic effects against Colorado potato beetle. |
| Targets | COX | NF-kB | PKC | p38MAPK |
| In vitro | Antitumor and antiplatelet activity of alkaloids from veratrum dahuricum.[Pubmed: 20013819 ]Phytother Res. 2010 Jun;24(6):821-6.
Cyclopamine and jervine induce COX-2 overexpression in human erythroleukemia cells but only cyclopamine has a pro-apoptotic effect.[Pubmed: 23357584 ]Exp Cell Res. 2013 Apr 15;319(7):1043-53.Erythroleukemia is generally associated with a very poor response and survival to current available therapeutic agents. Cyclooxygenase-2 (COX-2) has been described to play a crucial role in the proliferation and differentiation of leukemia cells, this enzyme seems to play an important role in chemoresistance in different cancer types. Previously, we demonstrated that diosgenin, a plant steroid, induced apoptosis in HEL cells with concomitant COX-2 overexpression. |
| Kinase Assay | Insecticidal metabolites from the rhizomes of Veratrum album against adults of Colorado potato beetle, Leptinotarsa decemlineata.[Pubmed: 25146763 ]Chem Biodivers. 2014 Aug;11(8):1192-204.The dried rhizomes of Veratrum album were individually extracted with CHCl3 , acetone, and NH4 OH/benzene to test the toxic effects against the Colorado potato beetle, Leptinotarsa decemlineata, which is an important agricultural pest. |

Jervine Dilution Calculator

Jervine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3496 mL | 11.7481 mL | 23.4962 mL | 46.9925 mL | 58.7406 mL |
| 5 mM | 0.4699 mL | 2.3496 mL | 4.6992 mL | 9.3985 mL | 11.7481 mL |
| 10 mM | 0.235 mL | 1.1748 mL | 2.3496 mL | 4.6992 mL | 5.8741 mL |
| 50 mM | 0.047 mL | 0.235 mL | 0.4699 mL | 0.9398 mL | 1.1748 mL |
| 100 mM | 0.0235 mL | 0.1175 mL | 0.235 mL | 0.4699 mL | 0.5874 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Inhibitor of Hedgehog signaling (IC50 = 500 - 700 nM); binds directly to Smoothened (Smo). Inhibits the response of target tissues to Sonic hedgehog signaling, possibly via the sterol sensing domain of Patched. Teratogenic.
- Cycloeucalenol
Catalog No.:BCN5519
CAS No.:469-39-6
- Hamamelitannin
Catalog No.:BCC8182
CAS No.:469-32-9
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- Cimilactone A
Catalog No.:BCN7948
CAS No.:468733-06-4
- 3-Benzofurancarboxaldehyde
Catalog No.:BCC8622
CAS No.:4687-25-6
- Dihydrocorynantheine
Catalog No.:BCN3747
CAS No.:4684-43-9
- Picrinine
Catalog No.:BCN5518
CAS No.:4684-32-6
- Norscopolamine
Catalog No.:BCN3983
CAS No.:4684-28-0
- Orphenadrine Citrate
Catalog No.:BCC4572
CAS No.:4682-36-4
- Drimenol
Catalog No.:BCN7224
CAS No.:468-68-8
- Mesembrenone
Catalog No.:BCN3753
CAS No.:468-54-2
- Lupulon
Catalog No.:BCC8204
CAS No.:468-28-0
- 5'-IMPdisodium salt
Catalog No.:BCN8175
CAS No.:4691-65-0
- Carbenicillin
Catalog No.:BCC5192
CAS No.:4697-36-3
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Isoalantolactone
Catalog No.:BCN4955
CAS No.:470-17-7
- Cinobufagin
Catalog No.:BCN5367
CAS No.:470-37-1
- Marinobufagin
Catalog No.:BCC9238
CAS No.:470-42-8
- Stachyose tetrahydrate
Catalog No.:BCC8252
CAS No.:470-55-3
- 1-Kestose
Catalog No.:BCN8292
CAS No.:470-69-9
- Cineole
Catalog No.:BCN2686
CAS No.:470-82-6
- Benzoyl-DL-methionine
Catalog No.:BCC8863
CAS No.:4703-38-2
- Beta-Lapachone
Catalog No.:BCC5088
CAS No.:4707-32-8
- alpha-Lapachone
Catalog No.:BCN5520
CAS No.:4707-33-9
Insecticidal metabolites from the rhizomes of Veratrum album against adults of Colorado potato beetle, Leptinotarsa decemlineata.[Pubmed:25146763]
Chem Biodivers. 2014 Aug;11(8):1192-204.
The dried rhizomes of Veratrum album were individually extracted with CHCl3 , acetone, and NH4 OH/benzene to test the toxic effects against the Colorado potato beetle, Leptinotarsa decemlineata, which is an important agricultural pest. Fifteen compounds in various amounts were isolated from the extracts using column and thin-layer chromatography. The chemical structures of 14 compounds were characterized as octacosan-1-ol (1), beta-sitosterol (2), stearic acid (3), diosgenin (4), resveratrol (5), wittifuran X (6), oxyresveratrol (7), beta-sitosterol 3-O-beta-D-glucopyranoside (8), diosgenin 3-O-alpha-L-rhamnopyranosyl-(1 --> 2)-beta-D-glucopyronoside (9), oxyresveratrol 3-O-beta-D-glucopyranoside (10), Jervine (11), pseudoJervine (13), 5,6-dihydro-1-hydroxyJervine (14), and saccharose (15) using UV, IR, MS, (1) H- and (13)C-NMR, and 2D-NMR spectroscopic methods. However, the chemical structure of 12, an oligosaccharide, has not fully been elucidated. Compounds 4, 6, 9, and 10 were isolated from V. album rhizomes for the first time in the current study. The toxic effects of three extracts (acetone, CHCl3 , and NH4 OH/benzene) and six metabolites, 2, 2+4, 5, 7, 8, and 11, were evaluated against the Colorado potato beetle. The assay revealed that all three extracts, and compounds 7, 8, and 11 exhibited potent toxic effects against this pest. This is the first report on the evaluation of the toxic effects of the extracts and secondary metabolites of V. album rhizomes against L. decemlineata. Based on these results, it can be concluded that the extracts can be used as natural insecticides.
Antitumor and antiplatelet activity of alkaloids from veratrum dahuricum.[Pubmed:20013819]
Phytother Res. 2010 Jun;24(6):821-6.
Ten steroidal alkaloids - cyclopamine, veratramine, Jervine, 3, 15-diangyloylgermine, 3-angyloylzygadenine, 3-veratroyl zygadenine, 15-veratroylgermine, germine, veratrosine and pseudoJervine - from Veratrum dahuricum, together with the ethanol extract and total alkaloids, were evaluated for their antitumor and antiplatelet activities. Cyclopamine, veratramine and germine significantly inhibited the hedgehog pathway in NIH/3T3 cells. Cyclopamine exerted a potent inhibitory effect against the growth of PANC-1 tumors in mice, with inhibition rates of 40.64%, 44.37%, 46.77% at doses of 5.0, 15.0 and 50.0 mg kg-1, respectively. Veratroylgermine was found to produce the strongest inhibition against the platelet aggregation induced by arachidonic acid, with inhibition rate of 92.0% at 100 microM.
Cyclopamine and jervine induce COX-2 overexpression in human erythroleukemia cells but only cyclopamine has a pro-apoptotic effect.[Pubmed:23357584]
Exp Cell Res. 2013 Apr 15;319(7):1043-53.
Erythroleukemia is generally associated with a very poor response and survival to current available therapeutic agents. Cyclooxygenase-2 (COX-2) has been described to play a crucial role in the proliferation and differentiation of leukemia cells, this enzyme seems to play an important role in chemoresistance in different cancer types. Previously, we demonstrated that diosgenin, a plant steroid, induced apoptosis in HEL cells with concomitant COX-2 overexpression. In this study, we investigated the antiproliferative and apoptotic effects of cyclopamine and Jervine, two steroidal alkaloids with similar structures, on HEL and TF1a human erythroleukemia cell lines and, for the first time, their effect on COX-2 expression. Cyclopamine, but not Jervine, inhibited cell proliferation and induced apoptosis in these cells. Both compounds induced COX-2 overexpression which was responsible for apoptosis resistance. In Jervine-treated cells, COX-2 overexpression was NF-kappaB dependent. Inhibition of NF-kappaB reduced COX-2 overexpression and induced apoptosis. In addition, cyclopamine induced apoptosis and COX-2 overexpression via PKC activation. Inhibition of the PKC pathway reduced both apoptosis and COX-2 overexpression in both cell lines. Furthermore, we demonstrated that the p38/COX-2 pathway was involved in resistance to cyclopamine-induced apoptosis since p38 inhibition reduced COX-2 overexpression and increased apoptosis in both cell lines.


