HamamelitanninCAS# 469-32-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 469-32-9 | SDF | Download SDF |
| PubChem ID | 471118 | Appearance | White powder |
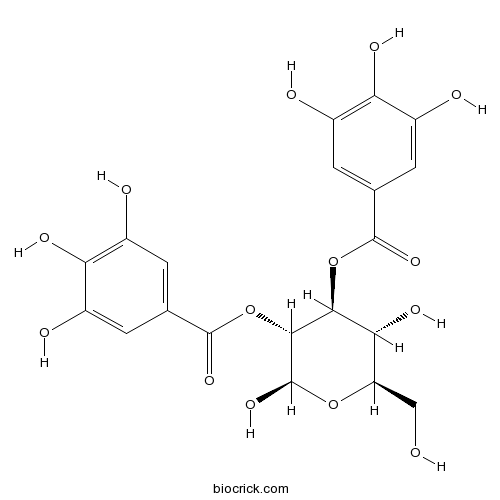
| Formula | C20H20O14 | M.Wt | 484.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | 2',5'-Di-O-galloyl D-hamamelose | ||
| Solubility | Soluble in ethanol and methanol; slightly soluble in water | ||
| Chemical Name | [(2R,3R,4S,5R,6R)-2,5-dihydroxy-6-(hydroxymethyl)-3-(3,4,5-trihydroxybenzoyl)oxyoxan-4-yl] 3,4,5-trihydroxybenzoate | ||
| SMILES | C1=C(C=C(C(=C1O)O)O)C(=O)OC2C(C(OC(C2OC(=O)C3=CC(=C(C(=C3)O)O)O)O)CO)O | ||
| Standard InChIKey | LRRLFFLVWQTQGZ-WRMYNCHHSA-N | ||
| Standard InChI | InChI=1S/C20H20O14/c21-5-12-15(28)16(33-18(29)6-1-8(22)13(26)9(23)2-6)17(20(31)32-12)34-19(30)7-3-10(24)14(27)11(25)4-7/h1-4,12,15-17,20-28,31H,5H2/t12-,15-,16+,17-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hamamelitannin has cytotoxic, and antibiofilm activities. It increases the susceptibility of S. aureus to antibiotic treatment in in vivo Caenorhabditis elegans and mouse mammary gland infection models. It also has a high protective activity on cell damage induced by peroxides. |
| Targets | Antifection |
| In vitro | Protective activity of hamamelitannin on cell damage induced by superoxide anion radicals in murine dermal fibroblasts.[Pubmed: 7735252]Biological & Pharmaceutical Bulletin, 1995, 18(1):59-63.
The Quorum Sensing Inhibitor Hamamelitannin Increases Antibiotic Susceptibility of Staphylococcus aureus Biofilms by Affecting Peptidoglycan Biosynthesis and eDNA Release.[Pubmed: 26828772 ]Sci Rep. 2016 Feb 1;6:20321.Treatment of Staphylococcus aureus infections has become increasingly challenging due to the rapid emergence and dissemination of methicillin-resistant strains. In addition, S. aureus reside within biofilms at the site of infection.
Few novel antibacterial agents have been developed in recent years and their bacteriostatic or bactericidal activity results in selective pressure, inevitably inducing antimicrobial resistance. Consequently, innovative antimicrobials with other modes of action are urgently needed. One alternative approach is targeting the bacterial quorum sensing (QS) system. Hamamelitannin (2',5-di-O-galloyl-d-hamamelose; HAM) was previously suggested to block QS through the TraP QS system and was shown to increase S. aureus biofilm susceptibility towards vancomycin (VAN) although mechanistic insights are still lacking. |
| In vivo | In vivo antibiofilm effect of cerium, chitosan and hamamelitannin against usual agents of catheter-related bloodstream infections.[Pubmed: 22991425]Journal of Antimicrobial Chemotherapy, 2013, 68(1):126-130.Catheter-related bloodstream infections (CRBSIs) are common healthcare-associated infections associated with increased morbidity and medical costs. Antiseptic- and antibiotic-coated central venous catheters (CVCs) have been proposed to reduce the incidence of CRBSIs, with variable success.
The aim of this study was to determine the in vivo antibiofilm activity of biocompatible and inexpensive compounds, such as cerium nitrate, chitosan and Hamamelitannin, against usual agents of CRBSIs. |
| Cell Research | Hamamelitannin from witch hazel (Hamamelis virginiana) displays specific cytotoxic activity against colon cancer cells.[Pubmed: 22216935]Journal of Natural Products, 2012, 75(1):26-33.Hamamelis virginiana (witch hazel) bark is a rich source of condensed and hydrolyzable tannins reported to exert a protective action against colon cancer. The present study characterizes different witch hazel tannins as selective cytotoxic agents against colon cancer. |

Hamamelitannin Dilution Calculator

Hamamelitannin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0644 mL | 10.322 mL | 20.6441 mL | 41.2882 mL | 51.6102 mL |
| 5 mM | 0.4129 mL | 2.0644 mL | 4.1288 mL | 8.2576 mL | 10.322 mL |
| 10 mM | 0.2064 mL | 1.0322 mL | 2.0644 mL | 4.1288 mL | 5.161 mL |
| 50 mM | 0.0413 mL | 0.2064 mL | 0.4129 mL | 0.8258 mL | 1.0322 mL |
| 100 mM | 0.0206 mL | 0.1032 mL | 0.2064 mL | 0.4129 mL | 0.5161 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- Cimilactone A
Catalog No.:BCN7948
CAS No.:468733-06-4
- 3-Benzofurancarboxaldehyde
Catalog No.:BCC8622
CAS No.:4687-25-6
- Dihydrocorynantheine
Catalog No.:BCN3747
CAS No.:4684-43-9
- Picrinine
Catalog No.:BCN5518
CAS No.:4684-32-6
- Norscopolamine
Catalog No.:BCN3983
CAS No.:4684-28-0
- Orphenadrine Citrate
Catalog No.:BCC4572
CAS No.:4682-36-4
- Drimenol
Catalog No.:BCN7224
CAS No.:468-68-8
- Mesembrenone
Catalog No.:BCN3753
CAS No.:468-54-2
- Lupulon
Catalog No.:BCC8204
CAS No.:468-28-0
- Colupulone
Catalog No.:BCN8097
CAS No.:468-27-9
- Lu AE58054
Catalog No.:BCC1707
CAS No.:467459-31-0
- Cycloeucalenol
Catalog No.:BCN5519
CAS No.:469-39-6
- Jervine
Catalog No.:BCN2975
CAS No.:469-59-0
- 5'-IMPdisodium salt
Catalog No.:BCN8175
CAS No.:4691-65-0
- Carbenicillin
Catalog No.:BCC5192
CAS No.:4697-36-3
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Isoalantolactone
Catalog No.:BCN4955
CAS No.:470-17-7
- Cinobufagin
Catalog No.:BCN5367
CAS No.:470-37-1
- Marinobufagin
Catalog No.:BCC9238
CAS No.:470-42-8
- Stachyose tetrahydrate
Catalog No.:BCC8252
CAS No.:470-55-3
- 1-Kestose
Catalog No.:BCN8292
CAS No.:470-69-9
- Cineole
Catalog No.:BCN2686
CAS No.:470-82-6
- Benzoyl-DL-methionine
Catalog No.:BCC8863
CAS No.:4703-38-2
Hamamelitannin from witch hazel (Hamamelis virginiana) displays specific cytotoxic activity against colon cancer cells.[Pubmed:22216935]
J Nat Prod. 2012 Jan 27;75(1):26-33.
Hamamelis virginiana (witch hazel) bark is a rich source of condensed and hydrolyzable tannins reported to exert a protective action against colon cancer. The present study characterizes different witch hazel tannins as selective cytotoxic agents against colon cancer. To cover the structural diversity of the tannins that occur in H. virginiana bark, the hydrolyzable tannins, Hamamelitannin and pentagalloylglucose, together with a proanthocyanidin-rich fraction (F800H4) were selected for the study. Treatment with these compounds reduced tumor viability and induced apoptosis, necrosis, and S-phase arrest in the cell cycle of HT29 cells, with Hamamelitannin being the most efficient. Owing to polyphenol-mediated H(2)O(2) formation in the incubation media, the antiproliferative effect was determined in the presence and absence of catalase to rule out any such interference. The presence of catalase significantly changed the IC(50) only for F800H4. Furthermore, at concentrations that inhibit the growth of HT29 cells by 50%, Hamamelitannin had no harmful effects on NCM460 normal colonocytes, whereas pentagalloylglucose inhibited both cancerous and normal cell growth. Using the TNPTM assay, we identified a highly reactive phenolic position in Hamamelitannin, which may explain its efficacy at inhibiting colon cancer growth.
Hamamelitannin Analogues that Modulate Quorum Sensing as Potentiators of Antibiotics against Staphylococcus aureus.[Pubmed:27095479]
Angew Chem Int Ed Engl. 2016 May 23;55(22):6551-5.
The modulation of bacterial communication to potentiate the effect of existing antimicrobial drugs is a promising alternative to the development of novel antibiotics. In the present study, we synthesized 58 analogues of Hamamelitannin (HAM), a quorum sensing inhibitor and antimicrobial potentiator. These efforts resulted in the identification of an analogue that increases the susceptibility of Staphylococcus aureus towards antibiotics in vitro, in Caenorhabditis elegans, and in a mouse mammary gland infection model, without showing cytotoxicity.
The Quorum Sensing Inhibitor Hamamelitannin Increases Antibiotic Susceptibility of Staphylococcus aureus Biofilms by Affecting Peptidoglycan Biosynthesis and eDNA Release.[Pubmed:26828772]
Sci Rep. 2016 Feb 1;6:20321.
Treatment of Staphylococcus aureus infections has become increasingly challenging due to the rapid emergence and dissemination of methicillin-resistant strains. In addition, S. aureus reside within biofilms at the site of infection. Few novel antibacterial agents have been developed in recent years and their bacteriostatic or bactericidal activity results in selective pressure, inevitably inducing antimicrobial resistance. Consequently, innovative antimicrobials with other modes of action are urgently needed. One alternative approach is targeting the bacterial quorum sensing (QS) system. Hamamelitannin (2',5-di-O-galloyl-d-hamamelose; HAM) was previously suggested to block QS through the TraP QS system and was shown to increase S. aureus biofilm susceptibility towards vancomycin (VAN) although mechanistic insights are still lacking. In the present study we provide evidence that HAM specifically affects S. aureus biofilm susceptibility through the TraP receptor by affecting cell wall synthesis and extracellular DNA release of S. aureus. We further provide evidence that HAM can increase the susceptibility of S. aureus biofilms towards different classes of antibiotics in vitro. Finally, we show that HAM increases the susceptibility of S. aureus to antibiotic treatment in in vivo Caenorhabditis elegans and mouse mammary gland infection models.
Dressings Loaded with Cyclodextrin-Hamamelitannin Complexes Increase Staphylococcus aureus Susceptibility Toward Antibiotics Both in Single as well as in Mixed Biofilm Communities.[Pubmed:26891369]
Macromol Biosci. 2016 Jun;16(6):859-69.
Bacteria reside within biofilms at the infection site, making them extremely difficult to eradicate with conventional wound care products. Bacteria use quorum sensing (QS) systems to regulate biofilm formation, and QS inhibitors (QSIs) have been proposed as promising antibiofilm agents. Despite this, few antimicrobial therapies that interfere with QS exist. Nontoxic hydroxypropyl-beta-cyclodextrin-functionalized cellulose gauzes releasing a burst of the antibiotic vancomycin and the QSI Hamamelitannin are developed, followed by a sustained release of both. The gauzes affect QS and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro model of chronic wound infection and can be considered as candidates to be used to prevent wound infection as well as treat infected wounds.
Novel hamamelitannin analogues for the treatment of biofilm related MRSA infections-A scaffold hopping approach.[Pubmed:27823882]
Eur J Med Chem. 2017 Feb 15;127:757-770.
Antimicrobial research is increasingly being focused on the problem of resistance and biofilm formation. Hamamelitannin (HAM) was recently identified as an antimicrobial potentiator for conventional antibiotics towards Staphylococcus aureus. This paper describes the synthesis and biological evaluation of novel Hamamelitannin analogues with alternative central scaffolds. Via a ligand-based approach, several interesting compounds with improved synthetic accessibility were identified as potentiators for vancomycin in the treatment of MRSA infections.


