CarbenicillinCAS# 4697-36-3 |
- Baicalein
Catalog No.:BCN5599
CAS No.:491-67-8
- Luteolin
Catalog No.:BCN5600
CAS No.:491-70-3
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- Vitamin D3
Catalog No.:BCN2186
CAS No.:67-97-0
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4697-36-3 | SDF | Download SDF |
| PubChem ID | 20824 | Appearance | Powder |
| Formula | C17H18N2O6S | M.Wt | 378.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
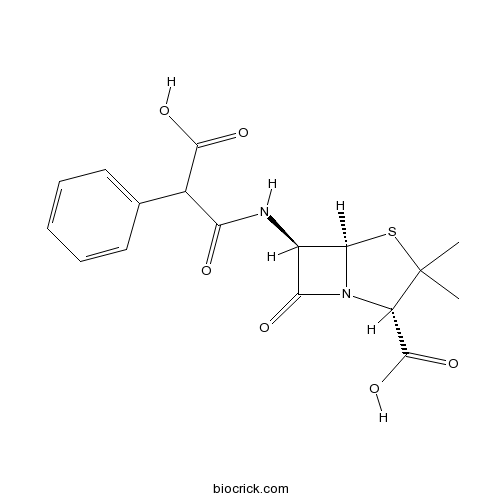
| Chemical Name | (2S,5R,6R)-6-[(2-carboxy-2-phenylacetyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid | ||
| SMILES | CC1(C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=CC=C3)C(=O)O)C(=O)O)C | ||
| Standard InChIKey | FPPNZSSZRUTDAP-UWFZAAFLSA-N | ||
| Standard InChI | InChI=1S/C17H18N2O6S/c1-17(2)11(16(24)25)19-13(21)10(14(19)26-17)18-12(20)9(15(22)23)8-6-4-3-5-7-8/h3-7,9-11,14H,1-2H3,(H,18,20)(H,22,23)(H,24,25)/t9?,10-,11+,14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Carbenicillin is broad-spectrum semisynthetic penicillin derivative used parenterally.
Target: Antibacterial
Carbenicillin is a semi-synthetic penicillin antibiotic which interferes with cell wall synthesis of gram-negative bacteria while displaying low toxicity. The leukocytes of the patients does not release histamine on in vitro provocation with Carbenicillin (0.1 g/mL). Carbenicillin (0.1 g/mL) does not show any allergic drug reactions in cystic fibrosis patients, as evident by no significant levels of antibodies of IgE, IgG or IgM classes [1]. Carbenicillin (50 μg/mL) results in phytotoxicity in chrysanthemum and TOB, with an increase in the concentration, and with a parallel shift in the morphogenic capacity (SRC) of threshold survival levels (TSLs). Carbenicillin results in 100% acclimatization with no different morphological flowering characteristics following subculture in vitro three times in Chrysanthemum plantlets [2]. References: | |||||

Carbenicillin Dilution Calculator

Carbenicillin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6427 mL | 13.2135 mL | 26.4271 mL | 52.8541 mL | 66.0677 mL |
| 5 mM | 0.5285 mL | 2.6427 mL | 5.2854 mL | 10.5708 mL | 13.2135 mL |
| 10 mM | 0.2643 mL | 1.3214 mL | 2.6427 mL | 5.2854 mL | 6.6068 mL |
| 50 mM | 0.0529 mL | 0.2643 mL | 0.5285 mL | 1.0571 mL | 1.3214 mL |
| 100 mM | 0.0264 mL | 0.1321 mL | 0.2643 mL | 0.5285 mL | 0.6607 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Carbenicillin is broad-spectrum semisynthetic penicillin derivative used parenterally.
- 5'-IMPdisodium salt
Catalog No.:BCN8175
CAS No.:4691-65-0
- Jervine
Catalog No.:BCN2975
CAS No.:469-59-0
- Cycloeucalenol
Catalog No.:BCN5519
CAS No.:469-39-6
- Hamamelitannin
Catalog No.:BCC8182
CAS No.:469-32-9
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- Cimilactone A
Catalog No.:BCN7948
CAS No.:468733-06-4
- 3-Benzofurancarboxaldehyde
Catalog No.:BCC8622
CAS No.:4687-25-6
- Dihydrocorynantheine
Catalog No.:BCN3747
CAS No.:4684-43-9
- Picrinine
Catalog No.:BCN5518
CAS No.:4684-32-6
- Norscopolamine
Catalog No.:BCN3983
CAS No.:4684-28-0
- Orphenadrine Citrate
Catalog No.:BCC4572
CAS No.:4682-36-4
- Drimenol
Catalog No.:BCN7224
CAS No.:468-68-8
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Isoalantolactone
Catalog No.:BCN4955
CAS No.:470-17-7
- Cinobufagin
Catalog No.:BCN5367
CAS No.:470-37-1
- Marinobufagin
Catalog No.:BCC9238
CAS No.:470-42-8
- Stachyose tetrahydrate
Catalog No.:BCC8252
CAS No.:470-55-3
- 1-Kestose
Catalog No.:BCN8292
CAS No.:470-69-9
- Cineole
Catalog No.:BCN2686
CAS No.:470-82-6
- Benzoyl-DL-methionine
Catalog No.:BCC8863
CAS No.:4703-38-2
- Beta-Lapachone
Catalog No.:BCC5088
CAS No.:4707-32-8
- alpha-Lapachone
Catalog No.:BCN5520
CAS No.:4707-33-9
- Atraric acid
Catalog No.:BCN5521
CAS No.:4707-47-5
- 8-Amino-7-oxononanoic acid
Catalog No.:BCN1778
CAS No.:4707-58-8
Inactivation of MuxABC-OpmB transporter system in Pseudomonas aeruginosa leads to increased ampicillin and carbenicillin resistance and decreased virulence.[Pubmed:21369987]
J Microbiol. 2011 Feb;49(1):107-14.
Resistance-Nodulation-Cell Division (RND) pumps play important roles in bacterial resistance to antibiotics. Pseudomonas aeruginosa is an important human pathogen which exhibits high level resistance to antibiotics. There are total of 12 RND pumps present in the P. aeruginosa PAOl genome. The recently characterized MuxABC-OpmB system has been shown to play a role in resistance to novobiocin, aztreonam, macrolides, and tetracycline in a multiple knockout mutation. In this study, we examined the expression levels of all the 12 RND pump gene clusters and tested the involvement of MuxABC-OpmB in pathogenicity. The results indicated that in addition to the four known constitutively expressed RND pumps, mexAB-oprM, mexGHI-opmD, mexVW, and mexXY, relatively high levels of expression were observed with mexJK and muxABC-opmB in the conditions tested. Inactivation of muxA in the muxABC-opmB operon resulted in elevated resistance to ampicillin and Carbenicillin. The mutant also showed attenuated virulence in both Brassica rapa pekinensis and Drosophila melanogaster infection models. The decreased virulence at least in part was due to decreased twitching motility in the mutant. These results indicate that the RND pump MuxABC-OpmB is associated with ampicillin and Carbenicillin susceptibility and also involved in pathogenesis in P. aeruginosa.
Effect of tannic and gallic acids alone or in combination with carbenicillin or tetracycline on Chromobacterium violaceum CV026 growth, motility, and biofilm formation.[Pubmed:26039903]
Can J Microbiol. 2015 Jul;61(7):487-94.
Chromobacterium violaceum is an opportunistic pathogen that causes infections that are difficult to treat. The goal of this research was to evaluate the effect of selected tannins (tannic acid (TA) and gallic acid (GA)) on bacterial growth, motility, antibiotic (Carbenicillin, tetracycline) susceptibility, and biofilm formation. Both tannins, particularly TA, impaired bacterial growth levels and swimming motilities at sub-minimum inhibitory concentrations (sub-MICs). In combination with tannins, antibiotics showed increased MICs, suggesting that tannins interfered with antibacterial activity. Sub-MICs of tetracycline or TA alone enhanced biofilm formation of C. violaceum; however, in combination, these compounds inhibited biofilm formation. In contrast, Carbenicillin at sub-MICs was effective in inhibiting C. violaceum biofilm formation; however, in combination with lower concentrations of TA or GA, biofilms were enhanced. These results provide insights into the effects of tannins on C. violaceum growth and their varying interaction with antibiotics used to target C. violaceum infections.
A polythiophene-derived ratiometric fluorescent sensor for highly sensitive determination of carbenicillin in aqueous solution.[Pubmed:22648337]
Chem Commun (Camb). 2012 Jul 11;48(54):6818-20.
A new ratiometric fluorescent sensor based on a quaternized quinine substituted 3-phenylpolythiophene derivative (PTQ1) was designed and developed for facile and reliable detection of Carbenicillin with high sensitivity and selectivity.
A response regulator from a soil metagenome enhances resistance to the beta-lactam antibiotic carbenicillin in Escherichia coli.[Pubmed:25782011]
PLoS One. 2015 Mar 17;10(3):e0120094.
Functional metagenomic analysis of soil metagenomes is a method for uncovering as-yet unidentified mechanisms for antibiotic resistance. Here we report an unconventional mode by which a response regulator derived from a soil metagenome confers resistance to the beta-lactam antibiotic Carbenicillin in Escherichia coli. A recombinant clone (betalr16) harboring a 5,169 bp DNA insert was selected from a metagenomic library previously constructed from a remote Alaskan soil. The betalr16 clone conferred specific resistance to Carbenicillin, with limited increases in resistance to other tested antibiotics, including other beta-lactams (penicillins and cephalosporins), rifampin, ciprofloxacin, erythromycin, chloramphenicol, nalidixic acid, fusidic acid, and gentamicin. Resistance was more pronounced at 24 degrees C than at 37 degrees C. Zone-of-inhibition assays suggested that the mechanism of Carbenicillin resistance was not due to antibiotic inactivation. The DNA insert did not encode any genes known to confer antibiotic resistance, but did have two putative open reading frames (ORFs) that were annotated as a metallopeptidase and a two-component response regulator. Transposon mutagenesis and subcloning of the two ORFs followed by phenotypic assays showed that the response regulator gene was necessary and sufficient to confer the resistance phenotype. Quantitative reverse transcriptase PCR showed that the response regulator suppressed expression of the ompF porin gene, independently of the small RNA regulator micF, and enhanced expression of the acrD, mdtA, and mdtB efflux pump genes. This work demonstrates that antibiotic resistance can be achieved by the modulation of gene regulation by heterologous DNA. Functional analyses such as these can be important for making discoveries in antibiotic resistance gene biology and ecology.


