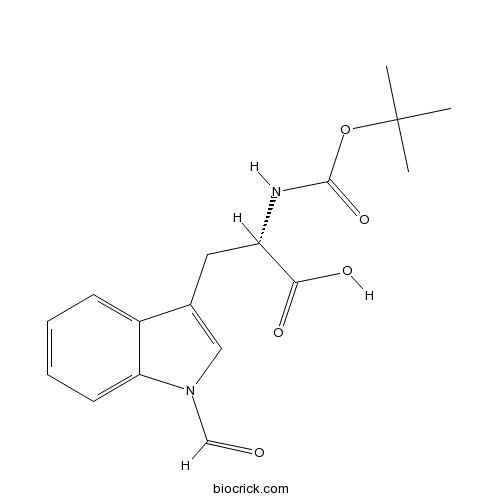
Boc-Trp(For)-OHCAS# 47355-10-2 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 47355-10-2 | SDF | Download SDF |
| PubChem ID | 7017963 | Appearance | Powder |
| Formula | C17H20N2O5 | M.Wt | 332.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-3-(1-formylindol-3-yl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid | ||
| SMILES | CC(C)(C)OC(=O)NC(CC1=CN(C2=CC=CC=C21)C=O)C(=O)O | ||
| Standard InChIKey | IHXHBYFWSOYYTR-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C17H20N2O5/c1-17(2,3)24-16(23)18-13(15(21)22)8-11-9-19(10-20)14-7-5-4-6-12(11)14/h4-7,9-10,13H,8H2,1-3H3,(H,18,23)(H,21,22)/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Trp(For)-OH Dilution Calculator

Boc-Trp(For)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0084 mL | 15.0421 mL | 30.0842 mL | 60.1685 mL | 75.2106 mL |
| 5 mM | 0.6017 mL | 3.0084 mL | 6.0168 mL | 12.0337 mL | 15.0421 mL |
| 10 mM | 0.3008 mL | 1.5042 mL | 3.0084 mL | 6.0168 mL | 7.5211 mL |
| 50 mM | 0.0602 mL | 0.3008 mL | 0.6017 mL | 1.2034 mL | 1.5042 mL |
| 100 mM | 0.0301 mL | 0.1504 mL | 0.3008 mL | 0.6017 mL | 0.7521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Trp(For)-OH
- Carpinontriol B
Catalog No.:BCN8113
CAS No.:473451-73-9
- Spegatrine
Catalog No.:BCN4068
CAS No.:47326-53-4
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Tolbutamide Sodium
Catalog No.:BCC5632
CAS No.:473-41-6
- beta-Eudesmol
Catalog No.:BCN6294
CAS No.:473-15-4
- alpha-Cyperone
Catalog No.:BCN1193
CAS No.:473-08-5
- SB 366791
Catalog No.:BCC7128
CAS No.:472981-92-3
- 8(14),15-Isopimaradien-3-ol
Catalog No.:BCN5526
CAS No.:4728-30-7
- 1-Benzyl-4-hydroxypiperidine
Catalog No.:BCC8459
CAS No.:4727-72-4
- Kartogenin
Catalog No.:BCC6211
CAS No.:4727-31-5
- H-Ser(Bzl)-OH
Catalog No.:BCC3031
CAS No.:4726-96-9
- Astaxanthin
Catalog No.:BCN2248
CAS No.:472-61-7
- AMG 487
Catalog No.:BCC5140
CAS No.:473719-41-4
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- SCH 563705
Catalog No.:BCC1933
CAS No.:473728-58-4
- Boc-Tyr(tBu)-OH
Catalog No.:BCC3462
CAS No.:47375-34-8
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Forskolin G
Catalog No.:BCN5527
CAS No.:473981-11-2
- Brazilin
Catalog No.:BCN5529
CAS No.:474-07-7
- Chenodeoxycholic acid
Catalog No.:BCN2620
CAS No.:474-25-9
- Citrostadienol
Catalog No.:BCN7357
CAS No.:474-40-8
- Reserpin N-oxide
Catalog No.:BCN3493
CAS No.:474-48-6
- Daucosterol
Catalog No.:BCN5531
CAS No.:474-58-8
- Campestanol
Catalog No.:BCN3890
CAS No.:474-60-2
Characterization of folded conformations in a tetrapeptide containing two tryptophan residues by vibrational circular dichroism.[Pubmed:19750497]
Chirality. 2009;21 Suppl 1:E76-85.
The intramolecularly hydrogen bonded conformations of the tetrapeptide Boc-Trp-Aib-Gly-Trp-OMe (WUGW) are investigated using experimental and quantum chemical predictions of vibrational circular dichroism (VCD) in the 1800-1550 cm(-1) region. The predicted VCD spectrum, for a conformation (conformer A) obtained from optimization of crystal structure, reproduced the dominant negative VCD band observed experimentally in CH(3)OH and CHCl(3) solvents. However, the predicted VCD spectrum of Conformation A also has an extra positive band which is not seen in the experimental spectra. This mismatch appears to be due to the lack of solvent influence in the quantum chemical geometry optimizations. However, Conformations I and II, obtained, respectively, from constrained optimization of crystal and NMR structures, mimic the solvent stabilized structures and are predicted to have dominant negative VCD band as found in the experimental spectra. It is noted that, for the peptide investigated here, unconstrained quantum chemical geometry optimizations in vacuum converged to structures that are not the realistic models of conformations found in solution. It is also noted that undertaking quantum chemical vibrational property calculations directly using geometries obtained from crystal data or NMR data resulted in unrealistic vibrational frequencies and descriptions. However, constraining the backbone dihedral angles to those found in condensed medium, and optimizing the remaining geometrical parameters resulted in a better reproduction of the observed VCD in condensed medium. The vibrational origins of bands in all of the predicted VCD spectra for the WUGW-tetrapeptide have also been presented.
Neurotensin modulates the electrical activity of frog pituitary melanotropes via activation of a G-protein-coupled receptor pharmacologically related to both the NTS1 and nts2 receptors of mammals.[Pubmed:11146421]
Neuroendocrinology. 2000 Dec;72(6):379-91.
The primary structure of frog neurotensin (fNT) has recently been determined and it has been shown that fNT is a potent stimulator of alpha-MSH secretion by frog pituitary melanotropes. In the present study, we have investigated the effects of fNT on the electrical activity of cultured frog melanotropes by using the patch-clamp technique and we have determined the pharmacological profile of the receptors mediating the effect of fNT. In the cell-attached configuration, fNT (10(-7) M) provoked an increase in the action current discharge followed by an arrest of spike firing. In the gramicidin-perforated patch configuration, fNT (10(-7) M) induced a depolarization accompanied by an increase in action potential frequency and a decrease in membrane resistance. Administration of graded concentrations (10(-10) to 10(-6) M) of fNT or the C-terminal hexapeptide NT(8-13) caused a dose-dependent increase in the frequency of action potentials with EC(50) of 2 x 10(-8) and 5 x 10(-9) M, respectively. The stimulatory effect of fNT was mimicked by various pseudopeptide analogs, with the following order of potency: Boc-[Trp(11)]NT(8-13) > Boc-[D-Trp(11)]NT(8-13) > Boc-[Lys(8,9), Nal(11)]NT(8-13) > Boc-[Psi11,12]NT(8-13). In contrast, the cyclic pseudopeptide analogs of NT(8-13), Lys-Lys-Pro-D-Trp-Ile-Leu and Lys-Lys-Pro-D-Trp-Glu-Leu-OH, did not affect the electrical activity. The NTS1 receptor antagonist and nts2 receptor agonist SR 48692 (10(-5) M) stimulated the spike discharge but did not block the response to fNT. In contrast, SR 142948A (10(-5) M), another NTS1 receptor antagonist and nts2 receptor agonist, inhibited the excitatory effect of fNT. The specific nts2 receptor ligand levocabastine (10(-6) M) had no effect on the basal electrical activity and the response of melanotropes to fNT. In cells which were dialyzed with guanosine-5'-O-(3-thiotriphosphate) (10(-4) M), fNT caused an irreversible stimulation of the action potential discharge. Conversely, dialysis of melanotropes with guanosine-5'-O-(2-thiodiphosphate) (10(-4) M) completely blocked the effect of fNT. Pretreatment of cells with cholera toxin (1 microg/ml) or pertussis toxin (0.2 microg/ml) did not affect the electrical response to fNT. Intracellular application of the G(o/i/s) protein antagonist GPAnt-1 (3 x 10(-5) M) had no effect on the fNT-evoked stimulation. In contrast, dialysis of melanotropes with the G(q/11) protein antagonist GPAnt-2A (3 x 10(-5) M) abrogated the response to fNT. The present data demonstrate that fNT is a potent stimulator of the electrical activity of frog pituitary melanotropes. These results also reveal that the electrophysiological response evoked by fNT can be accounted for by activation of a G(q/11)-protein-coupled receptor subtype whose pharmacological profile shares similarities with those of mammalian NTS1 and nts2 receptors.


