Z-WEHD-FMKCaspase 5 inhibitor,potent,cell-permeable and irreversible CAS# 210345-00-9 |
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- Z-IETD-FMK
Catalog No.:BCC5116
CAS No.:210344-98-2
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 210345-00-9 | SDF | Download SDF |
| PubChem ID | 25108687 | Appearance | Powder |
| Formula | C37H42FN7O10 | M.Wt | 763.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
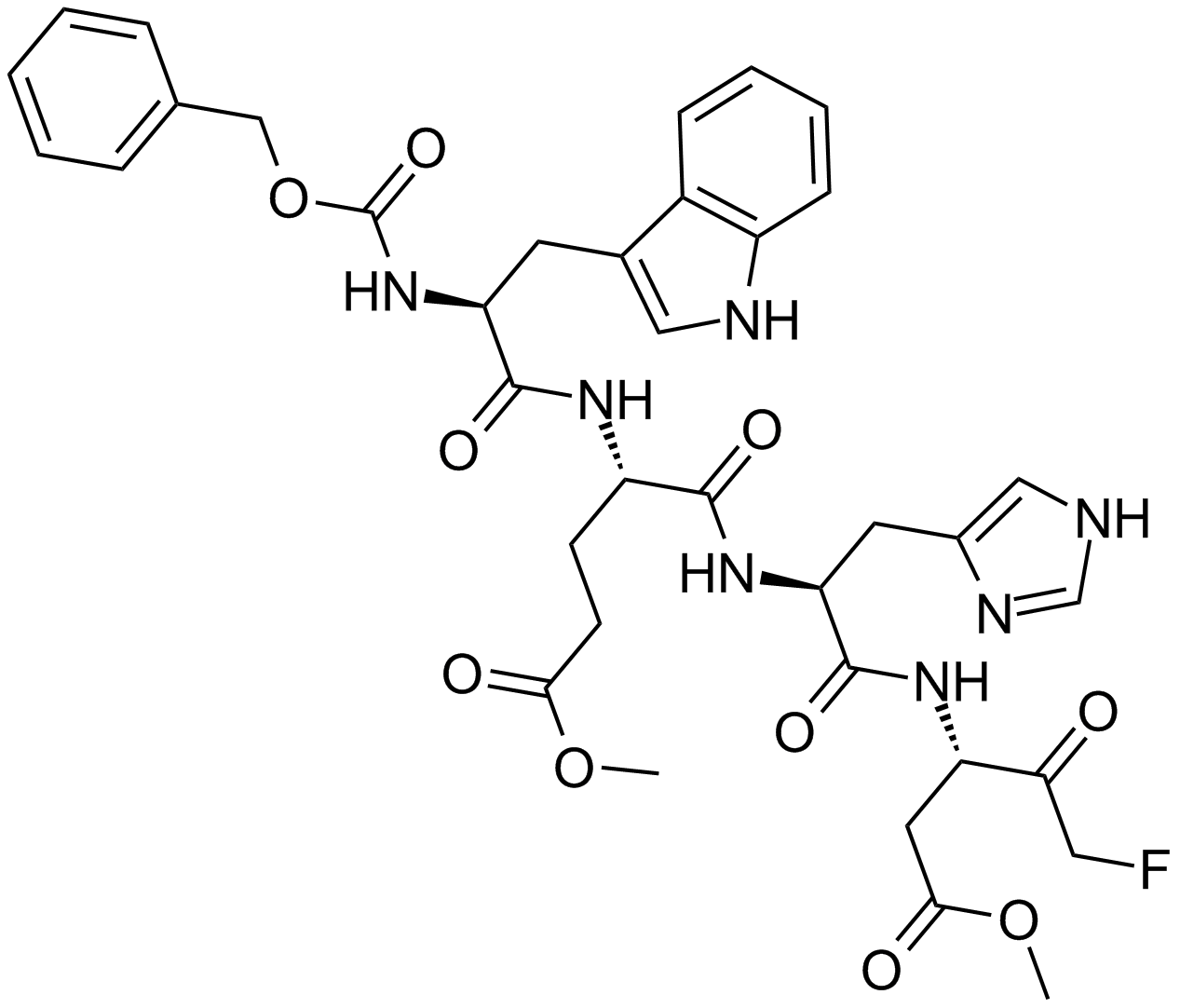
| Sequence | Z-Trp-Glu(OMe)-His-Asp(OMe)-FMK | ||
| Chemical Name | methyl (4S)-5-[[(2S)-1-[[(3S)-5-fluoro-1-methoxy-1,4-dioxopentan-3-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-4-[[(2S)-3-(1H-indol-3-yl)-2-(phenylmethoxycarbonylamino)propanoyl]amino]-5-oxopentanoate | ||
| SMILES | COC(=O)CCC(C(=O)NC(CC1=CN=CN1)C(=O)NC(CC(=O)OC)C(=O)CF)NC(=O)C(CC2=CNC3=CC=CC=C32)NC(=O)OCC4=CC=CC=C4 | ||
| Standard InChIKey | NLZNSSWGRVBWIX-KRCBVYEFSA-N | ||
| Standard InChI | InChI=1S/C37H42FN7O10/c1-53-32(47)13-12-27(34(49)44-30(15-24-19-39-21-41-24)36(51)43-28(31(46)17-38)16-33(48)54-2)42-35(50)29(14-23-18-40-26-11-7-6-10-25(23)26)45-37(52)55-20-22-8-4-3-5-9-22/h3-11,18-19,21,27-30,40H,12-17,20H2,1-2H3,(H,39,41)(H,42,50)(H,43,51)(H,44,49)(H,45,52)/t27-,28-,29-,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A synthetic peptide that irreversibly inhibits caspase-5 and related caspase activity. | |||||
| Targets | caspase-5 | |||||

Z-WEHD-FMK Dilution Calculator

Z-WEHD-FMK Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Treatment of infected cells with pan-caspase inhibitor IV and Z-WEHD-FMK, an inhibitor of inflammatory caspases, elicited a near-complete blockage of C. trachomatis-induced cleavage of golgin-84. Golgin-84 cleavage was blocked via treatment of infected cells with Z-WEHD-FMK, resulting in a lack of Golgi fragmentation and a 2-log reduction in numbers of infectious bacteria.
We treated infected cells with Z-WEHD-FMK, effectively preventing Golgi fragmentation, or with DMSO as a control and then labelled cells with fluorescent ceramide. Confocal images revealed that ceramide was rapidly incorporated into the inclusion membrane within DMSO-treated cells and accumulated inside the inclusion in bacterial membranes. In contrast, Z-WEHD-FMK-treated cells were only slightly fluorescent as the majority of lipid accumulated in a Golgi-like structure outside the inclusion1.
General caspase inhibitor (Z-Asp-CHz-DCB) and capase-5 inhibitor (Z-WEHD-FMK) could not induce rRNA fragmentation treated with ECyd. Caspase-5 (ICErei Ill/TY), member of ICE protease, activated pathway may be concerned with ECyd induced rRNA fragmentation2.
References:
1. D. Heuer, A.R. Lipinski et al. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. NATURE, 457, 2009
2. Kamada, S., Funahashi, Y. and Tsujimoto, Y.(1997) Cell Death Diff. 4, 473-478.
- Z-IETD-FMK
Catalog No.:BCC5116
CAS No.:210344-98-2
- Z-DEVD-FMK
Catalog No.:BCC1137
CAS No.:210344-95-9
- Gallein
Catalog No.:BCC7563
CAS No.:2103-64-2
- 7-Hydroxycadalene
Catalog No.:BCN7501
CAS No.:2102-75-2
- 6-Formyl-1,2,9,10-tetramethoxy-6a,7-dehydroaporphine
Catalog No.:BCN6436
CAS No.:2101836-45-5
- Amarogentin
Catalog No.:BCN2661
CAS No.:21018-84-8
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- 5,8,9,10,14-Pentaacetoxy-3-benzoyloxy-15-hydroxypepluane
Catalog No.:BCN1498
CAS No.:210108-91-1
- Jatrophane VI
Catalog No.:BCN7659
CAS No.:210108-90-0
- Jatrophane 5
Catalog No.:BCN1499
CAS No.:210108-89-7
- Jatrophane 4
Catalog No.:BCN1500
CAS No.:210108-88-6
- Jatrophane 3
Catalog No.:BCN1501
CAS No.:210108-87-5
- Ac-LEHD-AFC
Catalog No.:BCC2359
CAS No.:210345-03-2
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- Cinnamyl acetate
Catalog No.:BCN4914
CAS No.:21040-45-9
- Spiradine F
Catalog No.:BCN4915
CAS No.:21040-64-2
- Odoratin-7-O-beta-D-glucopyranoside
Catalog No.:BCN8089
CAS No.:210413-47-1
- Sitaxentan sodium
Catalog No.:BCC4495
CAS No.:210421-74-2
- 1,11b-Dihydro-11b-hydroxymedicarpin
Catalog No.:BCN3913
CAS No.:210537-04-5
- 1,11b-Dihydro-11b-hydroxymaackiain
Catalog No.:BCN3914
CAS No.:210537-05-6
- 6alpha-Hydroxylycopodine
Catalog No.:BCN7403
CAS No.:21061-92-7
- PD 168568 dihydrochloride
Catalog No.:BCC7702
CAS No.:210688-56-5
- 5,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8284
CAS No.:2107-76-8
- 7,8-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8290
CAS No.:2107-77-9
Overexpression of caspase 1 in apoptosis-resistant astrocytes infected with the BeAn Theiler's virus.[Pubmed:26567013]
J Neurovirol. 2016 Jun;22(3):316-26.
In this study, we demonstrate the upregulation in the expression of caspases 1 and 11 by SJL/J mouse brain astrocytes infected with the BeAn strain of Theiler's murine encephalomyelitis virus (TMEV). The upregulation of both proteases hints at protection of astrocytic cells from apoptotic death. We therefore looked for the reason of the demonstrated absence of programmed cell death in BeAn-infected SJL/J astrocytes. Complementary RNA (cRNA) from mock- and TMEV-infected cells was hybridized to the whole murine genome U74v2 DNA microarray from Affymetrix. Those experiments demonstrated the upregulation of gene expression for caspases 1 and 11 in infected cells. We further confirmed and validated their messenger RNA (mRNA) increase by reverse transcriptase quantitative real-time PCR (qPCR). The presence of both enzymatically active caspases 1 and 11 was demonstrated in cell lysates using a colorimetric and fluorymetric assay, respectively. We also show that overexpressed caspase 11 activated caspase 1 after preincubation of cytosol in vitro following a time-dependent process. This induction was neutralized by an anti-caspase 11 polyclonal antibody. These results demonstrate the activation of the caspase 1 precursor by caspase 11 and suggest a new mechanism of protection of BeAn-infected astrocytes from apoptosis. The direct experimental evidence that the protection effect demonstrated in this article was mediated by caspase 1, is provided by the fact that its specific inhibitor Z-WEHD-FMK induced de novo apoptotic death.
Inflammasome activation in airway epithelial cells after multi-walled carbon nanotube exposure mediates a profibrotic response in lung fibroblasts.[Pubmed:24915862]
Part Fibre Toxicol. 2014 Jun 10;11:28.
BACKGROUND: In vivo studies have demonstrated the ability of multi-walled carbon nanotubes (MWCNT) to induce airway remodeling, a key feature of chronic respiratory diseases like asthma and chronic obstructive pulmonary disease. However, the mechanism leading to remodeling is poorly understood. Particularly, there is limited insight about the role of airway epithelial injury in these changes. OBJECTIVES: We investigated the mechanism of MWCNT-induced primary human bronchial epithelial (HBE) cell injury and its contribution in inducing a profibrotic response. METHODS: Primary HBE cells were exposed to thoroughly characterized MWCNTs (1.5-24 mug/mL equivalent to 0.37-6.0 mug/cm2) and MRC-5 human lung fibroblasts were exposed to 1:4 diluted conditioned medium from these cells. Flow cytometry, ELISA, immunostainings/immunoblots and PCR analyses were employed to study cellular mechanisms. RESULTS: MWCNT induced NLRP3 inflammasome dependent pyroptosis in HBE cells in a time- and dose-dependent manner. Cell death and cytokine production were significantly reduced by antioxidants, siRNA to NLRP3, a caspase-1 inhibitor (Z-WEHD-FMK) or a cathepsin B inhibitor (CA-074Me). Conditioned medium from MWCNT-treated HBE cells induced significant increase in mRNA expression of pro-fibrotic markers (TIMP-1, Tenascin-C, Procollagen 1, and Osteopontin) in human lung fibroblasts, without a concomitant change in expression of TGF-beta. Induction of pro-fibrotic markers was significantly reduced when IL-1beta, IL-18 and IL-8 neutralizing antibodies were added to the conditioned medium or when conditioned medium from NLRP3 siRNA transfected HBE cells was used. CONCLUSIONS: Taken together these results demonstrate induction of a NLRP3 inflammasome dependent but TGF-beta independent pro-fibrotic response after MWCNT exposure.
Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice.[Pubmed:28394319]
Lab Invest. 2017 Aug;97(8):922-934.
Hyperhomocysteinemia (HHcy) has been shown to promote vascular inflammation and atherosclerosis, but the underlying mechanisms remain largely unknown. The NLRP3 inflammasome has been identified as the cellular machinery responsible for activation of inflammatory processes. In this study, we hypothesized that the activation of NLRP3 inflammasomes contributes to HHcy-induced inflammation and atherosclerosis. ApoE(-/-) mice were fed regular chow, high-fat (HF) diet, or HF plus high methionine diet to induce HHcy. To assess the role of NLRP3 inflammasomes in HHcy-aggravated atherosclerosis, NLRP3 shRNA viral suspension was injected via tail vein to knock down the NLRP3 gene. Increased plasma levels of IL-1beta and IL-18, aggravated macrophage infiltration into atherosclerotic lesions, and accelerated development of atherosclerosis were detected in HHcy mice as compared with control mice, and were associated with the activation of NLRP3 inflammasomes. Silencing the NLRP3 gene significantly suppressed NLRP3 inflammasome activation, reduced plasma levels of proinflammatory cytokines, attenuated macrophage infiltration and improved HHcy-induced atherosclerosis. We also examined the effect of homocysteine (Hcy) on NLRP3 inflammasome activation in THP-1-differentiated macrophages in the presence or absence of NLRP3 siRNA or the caspase-1 inhibitor Z-WEHD-FMK. We found that Hcy activated NLRP3 inflammasomes and promoted subsequent production of IL-1beta and IL-18 in macrophages, which were blocked by NLRP3 gene silencing or Z-WEHD-FMK. As reactive oxygen species (ROS) may have a central role in NLRP3 inflammasome activation, we next investigated whether antioxidant N-acetyl-l-cysteine (NAC) prevented Hcy-induced NLRP3 inflammasome activation in macrophages. We found Hcy-induced NLRP3 inflammasome activation was abolished by NAC. Treatment with NAC in HHcy mice also suppressed NLRP3 inflammasome activation and improved HHcy-induced atherosclerosis. These data suggest that the activation of NLRP3 inflammasomes contributes to HHcy-aggravated inflammation and atherosclerosis in apoE(-/-) mice. Hcy activates NLRP3 inflammasomes in ROS-dependent pathway in macrophages. These results may have implication for the treatment of HHcy-associated cardiovascular diseases.
Active caspase-1-mediated secretion of retinoic acid inducible gene-I.[Pubmed:18981155]
J Immunol. 2008 Nov 15;181(10):7324-31.
Caspase-1 is an inflammatory caspase that controls the activation and secretion of the inflammatory cytokines, IL-1beta and IL-18. We observed that cellular levels of retinoic acid-inducible gene-I (RIG-I) were enhanced when the pan-caspase inhibitor Z-VAD-fmk or caspase-1-specific inhibitor Z-WEHD-FMK blocked caspase activity. Overexpression of caspase-1 reduced cellular levels of RIG-I and inhibited RIG-I-mediated signaling activity. Enzymatic activity of caspase-1 was necessary to control RIG-I, although it was not a substrate of proteolytic cleavage by caspase-1. Caspase-1 physically interacted with full length RIG-I, but not with mutant forms lacking either the amino- or carboxyl-terminal domains. RIG-I was present in the supernatant of cells transfected with active caspase-1 but not with caspase-4. Stimulating cells with LPS and ATP also induced secretion of endogenous RIG-I in macrophages. Our data suggest a novel mechanism that negatively regulates RIG-I-mediated signaling activity via caspase-1-dependent secretion of RIG-I protein.
The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling.[Pubmed:18931327]
Am J Respir Cell Mol Biol. 2009 Apr;40(4):482-90.
Interleukin-1beta (IL-1beta), a proinflammatory cytokine, is elevated in cigarette smokers. To determine whether IL-1beta plays a role in the pathogenesis of cigarette smoke-induced emphysema and small airway remodeling, IL-1 receptor knockout (IL1RKO), TNF-alpha receptor knockout (TNFRKO), or C57Bl/6 (control) mice were exposed to cigarette smoke acutely or for up to 6 months. With a single acute exposure, smoke elevated IL-1beta in C57Bl/6 mice. IL1RKO mice were protected against acute smoke-mediated increases in lavage inflammatory cells and matrix breakdown. In C57Bl/6 mice, acute smoke-mediated increases in inflammatory cells, serum IL-1beta, and serum TNF-alpha were blocked by z-VAD-fmk, a pan-caspase inhibitor, or Z-WEHD-FMK, a caspase-1 (IL-1-converting enzyme, [ICE]) inhibitor. With 6 months of exposure, IL-1beta was no longer increased, but IL-18 was elevated. After 6 months of exposure, IL1RKO mice were 65% protected against emphysema, whereas TNFRKO mice were 83% protected. Both strains were completely protected against small airway remodeling. Lavage desmosine, hydroxyproline, and hyaluronan, matrix breakdown markers, were elevated in C57 but not IL1RKO mice. We conclude that IL-1beta plays a significant role in induction of murine emphysema and small airway remodeling, and is comparable to TNF-alpha in its effects. The protective effects of caspase inhibitors appear to be related to inhibition of ICE and raise the question of whether models that ameliorate emphysema with caspase inhibitors are really blocking IL-1beta (and IL-18) activation rather than blocking apoptosis.


