Jatrophane 4CAS# 210108-88-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 210108-88-6 | SDF | Download SDF |
| PubChem ID | 131851201 | Appearance | Powder |
| Formula | C39H52O14 | M.Wt | 744.8 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
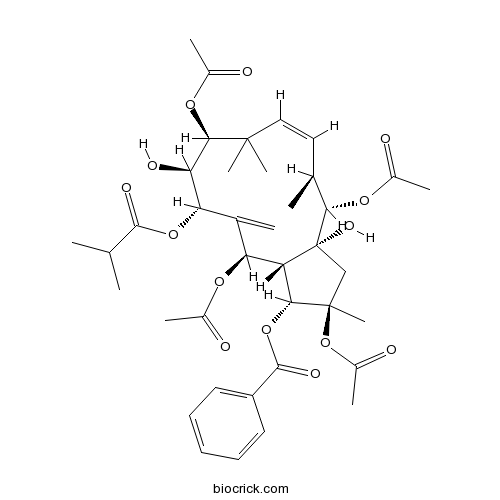
| Chemical Name | [(1R,2R,3aR,4S,5S,6Z,9S,10S,11S,13R,13aS)-2,4,9,13-tetraacetyloxy-3a,10-dihydroxy-2,5,8,8-tetramethyl-12-methylidene-11-(2-methylpropanoyloxy)-3,4,5,9,10,11,13,13a-octahydro-1H-cyclopenta[12]annulen-1-yl] benzoate | ||
| SMILES | CC1C=CC(C(C(C(C(=C)C(C2C(C(CC2(C1OC(=O)C)O)(C)OC(=O)C)OC(=O)C3=CC=CC=C3)OC(=O)C)OC(=O)C(C)C)O)OC(=O)C)(C)C | ||
| Standard InChIKey | WITHKWWZWFNDND-RAFXCFENSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (2R*,3R*,4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,9,14-Tetraacetoxy-3-benzoyloxy-8,15-dihydroxy-7-isobutyroyloxyjatropha-6(17),11E-diene(Jatrophane 4 ) and (2R*,3R*, 4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,14-triacetoxy-3-benzoyloxy-8,15-dihydroxy-7-isobutyroyloxy-9-nicotinoyloxyjatropha-6(17),11E-diene(Jatrophane 3) exhibit significant antifeedant activity against a generalist plant-feeding insect, the cotton bollworm (Helicoverpa armigera), with EC50 values of 0.36 and 0.43 ug/cm2, respectively. |
| Targets | Antifection |

Jatrophane 4 Dilution Calculator

Jatrophane 4 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3426 mL | 6.7132 mL | 13.4264 mL | 26.8528 mL | 33.5661 mL |
| 5 mM | 0.2685 mL | 1.3426 mL | 2.6853 mL | 5.3706 mL | 6.7132 mL |
| 10 mM | 0.1343 mL | 0.6713 mL | 1.3426 mL | 2.6853 mL | 3.3566 mL |
| 50 mM | 0.0269 mL | 0.1343 mL | 0.2685 mL | 0.5371 mL | 0.6713 mL |
| 100 mM | 0.0134 mL | 0.0671 mL | 0.1343 mL | 0.2685 mL | 0.3357 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Jatrophane 3
Catalog No.:BCN1501
CAS No.:210108-87-5
- Jatrophane 2
Catalog No.:BCN1502
CAS No.:210108-86-4
- Jatrophane I
Catalog No.:BCN7658
CAS No.:210108-85-3
- Nilgirine
Catalog No.:BCN2100
CAS No.:21009-05-2
- Erythbidin A
Catalog No.:BCN6859
CAS No.:210050-83-2
- LY-900009
Catalog No.:BCC2103
CAS No.:209984-68-9
- LY-411575 isomer 1
Catalog No.:BCC5443
CAS No.:209984-58-7
- LY-411575
Catalog No.:BCC2101
CAS No.:209984-57-6
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Androst-5-ene-3β,17β-diol 3,17-diacetate
Catalog No.:BCC8823
CAS No.:2099-26-5
- H-Phenylglycinol
Catalog No.:BCC2713
CAS No.:20989-17-7
- Resibufagin
Catalog No.:BCN8230
CAS No.:20987-24-0
- Jatrophane 5
Catalog No.:BCN1499
CAS No.:210108-89-7
- Jatrophane VI
Catalog No.:BCN7659
CAS No.:210108-90-0
- 5,8,9,10,14-Pentaacetoxy-3-benzoyloxy-15-hydroxypepluane
Catalog No.:BCN1498
CAS No.:210108-91-1
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Amarogentin
Catalog No.:BCN2661
CAS No.:21018-84-8
- 6-Formyl-1,2,9,10-tetramethoxy-6a,7-dehydroaporphine
Catalog No.:BCN6436
CAS No.:2101836-45-5
- 7-Hydroxycadalene
Catalog No.:BCN7501
CAS No.:2102-75-2
- Gallein
Catalog No.:BCC7563
CAS No.:2103-64-2
- Z-DEVD-FMK
Catalog No.:BCC1137
CAS No.:210344-95-9
- Z-IETD-FMK
Catalog No.:BCC5116
CAS No.:210344-98-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Ac-LEHD-AFC
Catalog No.:BCC2359
CAS No.:210345-03-2
Chemical profile and defensive function of the latex of Euphorbia peplus.[Pubmed:28062071]
Phytochemistry. 2017 Apr;136:56-64.
Plant latex is an endogenous fluid secreted from highly specialized laticifer cells and has been suggested to act as a plant defense system. The chemical profile of the latex of Euphorbia peplus was investigated. A total of 13 terpenoids including two previously unknown diterpenoids, (2S*,3S*,4R*,5R*,6R*,8R*,l1R*,13S*,14S*,15R*, 16R*)-5,8,15-triacetoxy-3-benzoyloxy-11,16-dihydroxy-9-oxopepluane and (2R*,3R*, 4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,8,9,14-pentaacetoxy-3-benzoyloxy-15-hydro xy-7-isobutyroyloxyjatropha-6(17),11E-diene), ten known diterpenoids, and a known acyclic triterpene alcohol peplusol, were identified, using HPLC and UPLC-MS/MS analyses and through comparison with the authentic compounds isolated from the whole plant. The diterpenoids exhibited significant antifeedant activity against a generalist plant-feeding insect, the cotton bollworm (Helicoverpa armigera), with EC50 values ranging from 0.36 to 4.60 mug/cm(2). In particular, (2R*,3R*,4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,9,14-tetraacetoxy-3-benzoyloxy-8 ,15-dihydroxy-7-isobutyroyloxyjatropha-6(17),11E-diene and (2R*,3R*, 4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,14-triacetoxy-3-benzoyloxy-8,15-dihydroxy -7-isobutyroyloxy-9-nicotinoyloxyjatropha-6(17),11E-diene had EC50 values of 0.36 and 0.43 mug/cm(2), respectively, which were approximately 7-fold more potent than commercial neem oil (EC50 = 2.62 mug/cm(2)). In addition, the major peplusol showed obvious antifungal activity against three strains of agricultural phytopathogenic fungi, Rhizoctonia solani, Colletotrichum litchi and Fusarium oxysporum f. sp. niveum. The results indicated that terpenoids in the latex of E. peplus are rich and highly diversified, and might function as constitutive defense metabolites against insect herbivores and pathogens for the plant.


