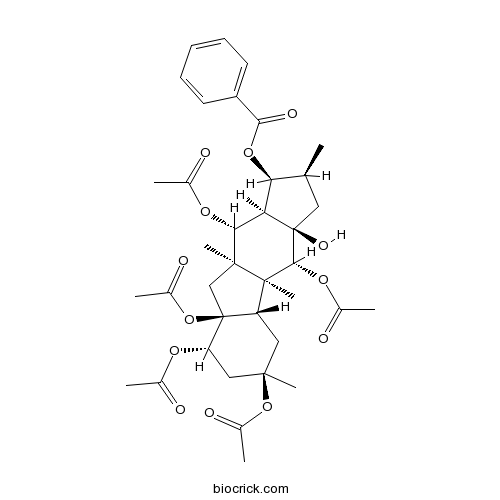
5,8,9,10,14-Pentaacetoxy-3-benzoyloxy-15-hydroxypepluaneCAS# 210108-91-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 210108-91-1 | SDF | Download SDF |
| PubChem ID | 10794828 | Appearance | Powder |
| Formula | C37H48O13 | M.Wt | 700.8 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,2S,3aR,4R,4aS,4bS,6R,8S,8aS,9aR,10R,10aR)-4,6,8,8a,10-pentaacetyloxy-3a-hydroxy-2,4a,6,9a-tetramethyl-2,3,4,4b,5,7,8,9,10,10a-decahydro-1H-cyclopenta[b]fluoren-1-yl] benzoate | ||
| SMILES | CC1CC2(C(C1OC(=O)C3=CC=CC=C3)C(C4(CC5(C(C4(C2OC(=O)C)C)CC(CC5OC(=O)C)(C)OC(=O)C)OC(=O)C)C)OC(=O)C)O | ||
| Standard InChIKey | IKVFCMXVZDVCLH-IEBUTITNSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Chinese Journal of Natural Medicines, 2010, 8(2):81-83.A New Jatrophane Diterpenoid from Euphorbia peplus.[Reference: WebLink]To study the chemical constituents of Euphorbia peplus. |

5,8,9,10,14-Pentaacetoxy-3-benzoyloxy-15-hydroxypepluane Dilution Calculator

5,8,9,10,14-Pentaacetoxy-3-benzoyloxy-15-hydroxypepluane Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4269 mL | 7.1347 mL | 14.2694 mL | 28.5388 mL | 35.6735 mL |
| 5 mM | 0.2854 mL | 1.4269 mL | 2.8539 mL | 5.7078 mL | 7.1347 mL |
| 10 mM | 0.1427 mL | 0.7135 mL | 1.4269 mL | 2.8539 mL | 3.5674 mL |
| 50 mM | 0.0285 mL | 0.1427 mL | 0.2854 mL | 0.5708 mL | 0.7135 mL |
| 100 mM | 0.0143 mL | 0.0713 mL | 0.1427 mL | 0.2854 mL | 0.3567 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Jatrophane VI
Catalog No.:BCN7659
CAS No.:210108-90-0
- Jatrophane 5
Catalog No.:BCN1499
CAS No.:210108-89-7
- Jatrophane 4
Catalog No.:BCN1500
CAS No.:210108-88-6
- Jatrophane 3
Catalog No.:BCN1501
CAS No.:210108-87-5
- Jatrophane 2
Catalog No.:BCN1502
CAS No.:210108-86-4
- Jatrophane I
Catalog No.:BCN7658
CAS No.:210108-85-3
- Nilgirine
Catalog No.:BCN2100
CAS No.:21009-05-2
- Erythbidin A
Catalog No.:BCN6859
CAS No.:210050-83-2
- LY-900009
Catalog No.:BCC2103
CAS No.:209984-68-9
- LY-411575 isomer 1
Catalog No.:BCC5443
CAS No.:209984-58-7
- LY-411575
Catalog No.:BCC2101
CAS No.:209984-57-6
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Amarogentin
Catalog No.:BCN2661
CAS No.:21018-84-8
- 6-Formyl-1,2,9,10-tetramethoxy-6a,7-dehydroaporphine
Catalog No.:BCN6436
CAS No.:2101836-45-5
- 7-Hydroxycadalene
Catalog No.:BCN7501
CAS No.:2102-75-2
- Gallein
Catalog No.:BCC7563
CAS No.:2103-64-2
- Z-DEVD-FMK
Catalog No.:BCC1137
CAS No.:210344-95-9
- Z-IETD-FMK
Catalog No.:BCC5116
CAS No.:210344-98-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Ac-LEHD-AFC
Catalog No.:BCC2359
CAS No.:210345-03-2
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- Cinnamyl acetate
Catalog No.:BCN4914
CAS No.:21040-45-9
- Spiradine F
Catalog No.:BCN4915
CAS No.:21040-64-2
Determination of the stereoisomers in aqueous medium and serum and validation studies of racemic aminoalkanol derivatives of 1,7-dimethyl-8,9-diphenyl-4-azatricyclo[5.2.1.0(2,6) ]dec-8-ene-3,5,10-trione, potential new anticancer drugs, by capillary electrophoresis.[Pubmed:27421088]
J Sep Sci. 2016 Aug;39(16):3246-53.
A new method for the determination of the stereoisomers, in aqueous medium and serum, of the racemic aminoalkanol derivatives I and II of 1,7-dimethyl-8,9-diphenyl-4-azatricyclo[5.2.1.0(2,6) ]dec-8-ene-3,5,10-trione, which were found in earlier studies to be potential anticancer drugs, was developed and validated. The optimized conditions included 25 mM phosphate buffer adjusted to pH 2.5, containing gamma-cyclodextrin at a concentration of 5% m/v, as background electrolyte, an applied voltage of +10 kV, and a temperature of 25 degrees C. Separations were carried out using a fused-silica capillary. The developed method of determining the enantiomers of compounds I(S), I(R) and II(S), II(R) was characterized by the following parameters: a detection time within 10.8 min, a detection limit in the range of 141.2-141.7 ng/mL using the UV absorption detection at 200 nm. Good linearity (R(2) = 0.9989-0.9998) was achieved within the range of concentrations studied. A very good extraction yield of 95.4-99.7% was achieved, and recoveries were carried out from both aqueous solutions and matrix serum. The repeatability of the method for peak areas with an accuracy of the determined concentrations of the analytes in the range of 1.43-1.89%, and limits of quantitation in the range of 432.4-436.3 ng/mL were achieved.
Crystal structure of (acetato-kappaO)(ethanol-kappaO)[(9S,17S,21S,29S)-9,17,21,29-tetra-hydroxy-18,30- dioxa-octa-cyclo-[18.10.0.0(2,7).0(8,19).0(9,17).0(11,16).0(21,29).0(23,28)]triac onta-1,3,5,7,11(16),12,14,19,23(28),24,26-undeca-ene-10,22-dione-kappa(3) O (18),O (21),O (22)]caesium ethanol monosolvate.[Pubmed:27555923]
Acta Crystallogr E Crystallogr Commun. 2016 Jun 3;72(Pt 7):884-7.
The title compound, [Cs(CH3COO)(C28H16O8)(C2H5OH)].C2H5OH, is the product of the complexation between one vasarene analogue [1], bis ninhydrin naphthalene-1,3-diol and CsF, where the F(-) ion has reacted with residual acetic acid (AcOH), to form a [1].CsOAc complex. The inter-molecular inter-actions with the multiple oxygen-containing functional groups of the ligand, as well as O-Hcdots, three dots, centeredO hydrogen bonds involving the ethanol solvent mol-ecules, stabilize the complex, forming a chain along [100]. Additional parallel-displaced pi-pi stacking, with an inter-planar distance of 3.669 (1) A, connect several unit cells in a three-dimensional supra-molecular structure, though, the larger size of AcO(-) (1.60 A) compared to F(-) (1.33 A) prevents the tight packing that was once achieved with other vasarene complexes of CsF.
Spectral investigation of the effect of anion on the stability of non covalent assemblies of 2,3,5,6,8,9,11,12-octahydro-1,4,7,10,13-benzopentaoxacyclopentadecine (benzo-15-crown-5) with sodium halides.[Pubmed:27591702]
Spectrochim Acta A Mol Biomol Spectrosc. 2017 Jan 15;171:507-514.
A series of complexes of 2,3,5,6,8,9,11,12-octahydro-1,4,7,10,13-benzopentaoxacyclopentadecine (benzo-15-crown-5) with sodium halides was synthesized in acetonitrile. The effect of anion on the stability and spectral properties of complexes of benzo-15-crown-5 with sodium halides was investigated. The synthesis of complexes of sodium fluoride and sodium chloride are reported for the first time. Chloroform was used as solvent to study the assembly in solution state by (1)H and (13)C NMR techniques. Single crystal diffraction studies on the easily crystallizable bromide complex confirmed 1:1 stoichiometry of the complex. IR and Raman studies provided valuable evidence for a water molecule shared between the crown encapsulated cation and the counter ion to give a solvent shared ion pair (SSIP). The fluorescence spectra of the complexes were obtained in chloroform by excitation at 270nm to study the effect of complexation on the fluorescent properties of benzo-15-crown-5.
Hybrid gels by conjugation of hyaluronic acid with poly(itaconic anhydride-co-3,9-divinyl-2,4,8,10-tetraoxaspiro (5.5)undecane) copolymers.[Pubmed:28153463]
Int J Biol Macromol. 2017 May;98:407-418.
The approach of covalent conjugation for coupling synthetic polymers with biomolecules represents an appealing strategy to produce new compounds with distinctive properties for biomedical applications. In the present study we generated hybrid gels with tunable characteristics by using hyaluronic acid (HA) and four variants of poly(itaconic anhydride-co-3,9-divinyl-2,4,8,10-tetraoxaspiro[5.5] undecane) (PITAU) copolymers, differing through the molar ratios between comonomers. The new bioconjugate compounds were realized by using a ''grafting to'' strategy, for further ensuring new ways for coupling of various bioactive compounds, taking into account that the grafted copolymers are dual sensitive to pH and temperature. The procedure of chemical crosslinking, by opening the anhydride cycle of the copolymer with the hydroxyl groups of hyaluronic acid, was used to prepare the bioconjugates. The chemical conjugation between HA and PITAU copolymers, as well as the structure of the new compounds, was confirmed by FTIR and NMR techniques. The physical properties of the new gels as thermal stability, swelling capacity, and rheological properties were investigated. The bioconjugate networks were also investigated as drug delivery carriers by using indomethacin as a model drug. In vitro and in vivo tests attested the homogeneity of the bioactive compounds as well as a good biochemical response, showing good biocompatibility for the new structures.


