Ac-LEHD-AFCFluorogenic caspase substrate CAS# 210345-03-2 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 210345-03-2 | SDF | Download SDF |
| PubChem ID | 44134612 | Appearance | Powder |
| Formula | C33H38F3N7O11 | M.Wt | 765.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | N-Acetyl-Leu-Glu-His-Asp-7-amino-4-Trifluoromethylcoumarin | ||
| Solubility | Soluble to 1 mg/ml in water | ||
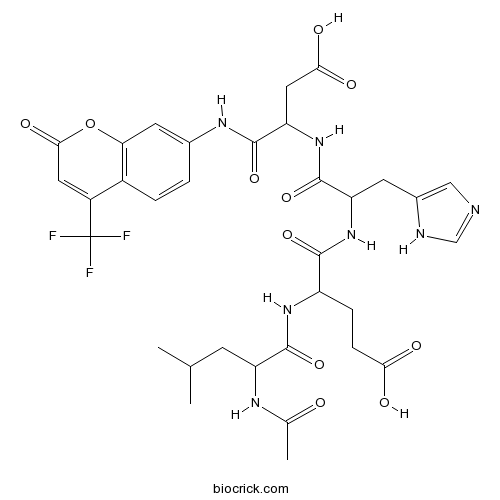
| Sequence | LEHD (Modifications: Leu-1 = N-Ac-Leu, Asp-4 = Asp-(7-amino-4-trifluoromethyl | ||
| Chemical Name | 4-[(2-acetamido-4-methylpentanoyl)amino]-5-[[1-[[3-carboxy-1-oxo-1-[[2-oxo-4-(trifluoromethyl)chromen-7-yl]amino]propan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-5-oxopentanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CCC(=O)O)C(=O)NC(CC1=CN=CN1)C(=O)NC(CC(=O)O)C(=O)NC2=CC3=C(C=C2)C(=CC(=O)O3)C(F)(F)F)NC(=O)C | ||
| Standard InChIKey | HULKIXRFKRCRHD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C33H38F3N7O11/c1-15(2)8-22(39-16(3)44)31(52)41-21(6-7-26(45)46)29(50)42-23(9-18-13-37-14-38-18)32(53)43-24(12-27(47)48)30(51)40-17-4-5-19-20(33(34,35)36)11-28(49)54-25(19)10-17/h4-5,10-11,13-15,21-24H,6-9,12H2,1-3H3,(H,37,38)(H,39,44)(H,40,51)(H,41,52)(H,42,50)(H,43,53)(H,45,46)(H,47,48) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fluorogenic caspase substrate. Analog of the caspase-9 substrate, LEHD-AFC. |

Ac-LEHD-AFC Dilution Calculator

Ac-LEHD-AFC Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ac-LEHD-AFC is a fluorogenic substrate that can be cleaved by caspase-4, -5, and -9.Caspase activity can be quantified by fluorescent detection of free AFC (also known as 7-amino-4-trifluoromethylcoumarin), which is excited at 400 nm and emits at 505 nm.
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-IETD-FMK
Catalog No.:BCC5116
CAS No.:210344-98-2
- Z-DEVD-FMK
Catalog No.:BCC1137
CAS No.:210344-95-9
- Gallein
Catalog No.:BCC7563
CAS No.:2103-64-2
- 7-Hydroxycadalene
Catalog No.:BCN7501
CAS No.:2102-75-2
- 6-Formyl-1,2,9,10-tetramethoxy-6a,7-dehydroaporphine
Catalog No.:BCN6436
CAS No.:2101836-45-5
- Amarogentin
Catalog No.:BCN2661
CAS No.:21018-84-8
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- 5,8,9,10,14-Pentaacetoxy-3-benzoyloxy-15-hydroxypepluane
Catalog No.:BCN1498
CAS No.:210108-91-1
- Jatrophane VI
Catalog No.:BCN7659
CAS No.:210108-90-0
- Jatrophane 5
Catalog No.:BCN1499
CAS No.:210108-89-7
- Jatrophane 4
Catalog No.:BCN1500
CAS No.:210108-88-6
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- Cinnamyl acetate
Catalog No.:BCN4914
CAS No.:21040-45-9
- Spiradine F
Catalog No.:BCN4915
CAS No.:21040-64-2
- Odoratin-7-O-beta-D-glucopyranoside
Catalog No.:BCN8089
CAS No.:210413-47-1
- Sitaxentan sodium
Catalog No.:BCC4495
CAS No.:210421-74-2
- 1,11b-Dihydro-11b-hydroxymedicarpin
Catalog No.:BCN3913
CAS No.:210537-04-5
- 1,11b-Dihydro-11b-hydroxymaackiain
Catalog No.:BCN3914
CAS No.:210537-05-6
- 6alpha-Hydroxylycopodine
Catalog No.:BCN7403
CAS No.:21061-92-7
- PD 168568 dihydrochloride
Catalog No.:BCC7702
CAS No.:210688-56-5
- 5,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8284
CAS No.:2107-76-8
- 7,8-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8290
CAS No.:2107-77-9
- CP 471474
Catalog No.:BCC2373
CAS No.:210755-45-6
Je-chun-jun induced apoptosis of human cervical carcinoma HeLa cells.[Pubmed:15456542]
Acta Pharmacol Sin. 2004 Oct;25(10):1372-9.
AIM: To study the mechanism of je-chun-jun (JCJ)-inducing the apoptosis of the human cervical carcinoma, HeLa cells. METHODS: The cell viability was assessed using MTT assay. The optical density was measured at 570 nm. The caspase activity was measured using 50 mmol/L of fluorogenic substrate, AC-DEVD-AMC (caspase-3), AC-VEID-AMC (caspase-8) or Ac-LEHD-AFC (caspase-9). To confirm the expression of proteins, Western blotting was performed. To detect the characteristic of apoptosis chromatin condensation, HeLa cells were stained with Hoechst 33258 in the presence of JCJ. For the cell cycle analysis, HeLa cells were incubated with Propidium iodide (PI) solution. Fluorescence intensity of cell cycle was measured using flow cytometry system. RESULTS: The loss of viability occurred following the exposure of 10 g/L JCJ. Cells treated with 10 g/L JCJ for 3 d exhibited the apoptotic morphology (brightly blue-fluorescent condensed nuclei by Hoechst 33258-staining) and the reduction of cell volume. Cells incubated with JCJ for 48 h were arrested at the G1 phase of cell cycle and their G1 checkpoint related gene products such as cyclin D1 were transiently decreased. We showed that JCJ induced the p38 MAPK activation in HeLa cells. The p38 MAPK inhibitor, SB203580 protected Hela cells from the JCJ-induced death as well as intervened the JCJ-induced accumulation of cells at the G1 phase. In contrast, MEK1 (-ERK upstream) inhibitor, PD98059 had no effect on HeLa cells. CONCLUSION: JCJ induced cell cycle arrest and apoptosis of HeLa cells through p38 MAPK pathway.
Molecular mechanisms of apoptosis induced by Scorpio water extract in human hepatoma HepG2 cells.[Pubmed:15742393]
World J Gastroenterol. 2005 Feb 21;11(7):943-7.
AIM: To clarify the mechanism underlying the anti-mutagenic and anti-cancer activities of Scorpio water extract (SWE). METHODS: Human hepatoma HepG2 cells were incubated with various concentrations of SWE. After 24-h incubation, cytotoxicity and apoptosis evaluations were determined by MTT and DNA fragmentation assay, respectively. After treatment with SWE, mitochondrial membrane potential (MMP) was determined by measuring the retention of the dye 3,3'-dihexyloxacarbocyanine (DiOC(6)(3)) and the protein expression including cytochrome C and poly-(ADP-ribose) polymerase (PARP) were measured by Western blotting. Caspase-3 and -9 enzyme activities were measured using specific fluorescence dyes such as Ac-DEVD-AFC and Ac-LEHD-AFC. RESULTS: We found that treatment with SWE induced apoptosis as confirmed by discontinuous DNA fragmentation in cultured human hepatoma HepG2 cells. Our investigation also showed that SWE-induced apoptosis of HepG2 cells were associated with intracellular events including disruption of MMP, increased translocation of cytochrome C from mitochondria to cytosol, activation of caspase-3, and PARP. Pre-treatment of N-acetyl-Asp-Glu-Val-Asp-CHO (Ac-DEVD-CHO), a caspase-3 specific inhibitor, or cyclosporin A (CsA), an inhibitor of MMP disruption, completely abolished SWE-induced DNA fragmentation. CONCLUSION: These results suggest that SWE possibly causes mitochondrial damage, leading to cytochrome C release into cytosol and activation of caspases resulting in PARP cleavage and execution of apoptotic cell death in HepG2 cells. These results further suggest that Scorpio may be a valuable agent of therapeutic intervention of human hepatomas.
Mechanism of nitric oxide-induced apoptosis in human neuroblastoma SH-SY5Y cells.[Pubmed:11078888]
FEBS Lett. 2000 Nov 10;484(3):253-60.
We have attempted to elucidate the precise mechanism of nitric oxide (NO)-induced apoptotic neuronal cell death. Enzymatic cleavages of DEVD-AFC, VDVAD-AFC, and LEHD-AFC (specific substrates for caspase-3-like protease (caspase-3 and -7), caspase-2, and caspase-9, respectively) were observed by treatment with NO. Western blot analysis showed that pro-forms of caspase-2, -3, -6, and -7 are decreased during apoptosis. Interestingly, Ac-DEVD-CHO, a caspase-3-like protease inhibitor, blocked not only the decreases in caspase-2 and -7, but also the formation of p17 from p20 in caspase-3 induced by NO, suggesting that caspase-3 exists upstream of caspase-2 and -7. Bongkrekic acid, a potent inhibitor of mitochondrial permeability transition, specifically blocked both the loss of mitochondrial membrane potential and subsequent DNA fragmentation in response to NO. Thus, NO results in neuronal apoptosis through the sequential loss of mitochondrial membrane potential, caspase activation, and degradation of inhibitor of caspase-activated DNase (CAD) (CAD activation).
Possible involvement of cytochrome c release and sequential activation of caspases in ceramide-induced apoptosis in SK-N-MC cells.[Pubmed:10590315]
Biochim Biophys Acta. 1999 Dec 9;1452(3):263-74.
Ceramide is characterized as a second messenger of apoptosis induced by various agents such as tumor necrosis factor (TNF-alpha), Fas ligand, hydrogen peroxide, heat shock and ionizing radiation. In this study, we investigated the mechanism of ceramide-induced apoptosis using a human neuroblastoma cell line, SK-N-MC. N-Acetyl-sphingosine (C2-ceramide), a cell-permeable ceramide analogue, was able to induce apoptosis in SK-N-MC cells as estimated by DNA fragmentation and chromatin condensation. C2-ceramide-induced DNA fragmentation was blocked by caspase inhibitor (Z-Asp-CH(2)-DCB). An increase in caspase-3 (CPP32)-like protease activity was evident during C2-ceramide-induced apoptosis, suggesting that caspases are involved in this apoptosis. Moreover, enzymatic cleavage of VDVAD-AFC and LEHD-AFC (specific substrates for caspase-2 and -9, respectively) was increased by treatment with C2-ceramide. To elucidate which types of caspase are activated in C2-ceramide-treated cells, we performed Western blot analysis using antibodies against each isoform. Both proforms of caspase-2 and -3 were decreased in response to C2-ceramide in a time-dependent manner. Mitochondrial cytochrome c is also time-dependently released into the cytosol in response to treatment with C2-ceramide. Addition of cytochrome c into the S-100 fractions prepared from SK-N-MC cells could activate caspase-2 in cell-free systems. These results suggest the possibility that cytochrome c released to the cytosol can activate caspases (caspase-9, -3, and -2) during C2-ceramide-induced apoptosis of SK-N-MC cells.


