Sitaxentan sodiumCAS# 210421-74-2 |
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
- Clozapine
Catalog No.:BCC5037
CAS No.:5786-21-0
- LY404039
Catalog No.:BCC4592
CAS No.:635318-11-5
- Amisulpride
Catalog No.:BCC4459
CAS No.:71675-85-9
- Amisulpride hydrochloride
Catalog No.:BCC4252
CAS No.:81342-13-4
- Brexpiprazole
Catalog No.:BCC4118
CAS No.:913611-97-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 210421-74-2 | SDF | Download SDF |
| PubChem ID | 11477084 | Appearance | Powder |
| Formula | C18H14ClN2NaO6S2 | M.Wt | 476.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (209.69 mM; Need ultrasonic) | ||
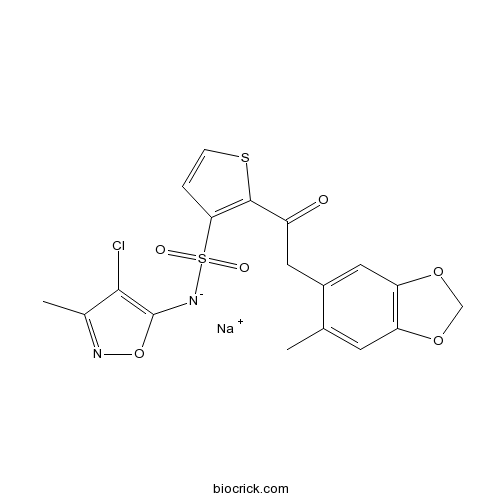
| Chemical Name | sodium;(4-chloro-3-methyl-1,2-oxazol-5-yl)-[2-[2-(6-methyl-1,3-benzodioxol-5-yl)acetyl]thiophen-3-yl]sulfonylazanide | ||
| SMILES | CC1=CC2=C(C=C1CC(=O)C3=C(C=CS3)S(=O)(=O)[N-]C4=C(C(=NO4)C)Cl)OCO2.[Na+] | ||
| Standard InChIKey | MDTNUYUCUYPIHE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H14ClN2O6S2.Na/c1-9-5-13-14(26-8-25-13)7-11(9)6-12(22)17-15(3-4-28-17)29(23,24)21-18-16(19)10(2)20-27-18;/h3-5,7H,6,8H2,1-2H3;/q-1;+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sitaxentan sodium Dilution Calculator

Sitaxentan sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0969 mL | 10.4846 mL | 20.9692 mL | 41.9384 mL | 52.423 mL |
| 5 mM | 0.4194 mL | 2.0969 mL | 4.1938 mL | 8.3877 mL | 10.4846 mL |
| 10 mM | 0.2097 mL | 1.0485 mL | 2.0969 mL | 4.1938 mL | 5.2423 mL |
| 50 mM | 0.0419 mL | 0.2097 mL | 0.4194 mL | 0.8388 mL | 1.0485 mL |
| 100 mM | 0.021 mL | 0.1048 mL | 0.2097 mL | 0.4194 mL | 0.5242 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sitaxsentan sodium (TBC11251 sodium salt) is an orally active, highly selective antagonist of endothelin A receptors.
- Odoratin-7-O-beta-D-glucopyranoside
Catalog No.:BCN8089
CAS No.:210413-47-1
- Spiradine F
Catalog No.:BCN4915
CAS No.:21040-64-2
- Cinnamyl acetate
Catalog No.:BCN4914
CAS No.:21040-45-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- Ac-LEHD-AFC
Catalog No.:BCC2359
CAS No.:210345-03-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-IETD-FMK
Catalog No.:BCC5116
CAS No.:210344-98-2
- Z-DEVD-FMK
Catalog No.:BCC1137
CAS No.:210344-95-9
- Gallein
Catalog No.:BCC7563
CAS No.:2103-64-2
- 7-Hydroxycadalene
Catalog No.:BCN7501
CAS No.:2102-75-2
- 6-Formyl-1,2,9,10-tetramethoxy-6a,7-dehydroaporphine
Catalog No.:BCN6436
CAS No.:2101836-45-5
- Amarogentin
Catalog No.:BCN2661
CAS No.:21018-84-8
- 1,11b-Dihydro-11b-hydroxymedicarpin
Catalog No.:BCN3913
CAS No.:210537-04-5
- 1,11b-Dihydro-11b-hydroxymaackiain
Catalog No.:BCN3914
CAS No.:210537-05-6
- 6alpha-Hydroxylycopodine
Catalog No.:BCN7403
CAS No.:21061-92-7
- PD 168568 dihydrochloride
Catalog No.:BCC7702
CAS No.:210688-56-5
- 5,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8284
CAS No.:2107-76-8
- 7,8-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8290
CAS No.:2107-77-9
- CP 471474
Catalog No.:BCC2373
CAS No.:210755-45-6
- Sakakin
Catalog No.:BCN4916
CAS No.:21082-33-7
- Org 12962 hydrochloride
Catalog No.:BCC7718
CAS No.:210821-63-9
- W-84 dibromide
Catalog No.:BCC6682
CAS No.:21093-51-6
- CART (62-76) (rat, human)
Catalog No.:BCC6008
CAS No.:210978-19-1
- BMY 7378
Catalog No.:BCC5063
CAS No.:21102-95-4
An evaluation of reproductive and developmental toxicity of sitaxentan (thelin) in rats.[Pubmed:22890981]
Birth Defects Res B Dev Reprod Toxicol. 2012 Oct;95(5):327-36.
Sitaxentan sodium (Thelin) is a once daily, orally bioavailable, highly selective endothelin A receptor antagonist. Initially approved for the treatment of pulmonary arterial hypertension, sitaxentan was withdrawn in 2010 following the recognition of a pattern of idiosyncratic liver injury. During development of this drug, a series of nonclinical studies investigated the effects of orally administered sitaxentan on fertility, embryofetal development, and pre- and postnatal development in the rat; results of these studies are reported here. In the fertility study, sitaxentan did not affect mating behavior, fertility, sperm morphology, or estrous cycle. Sitaxentan was teratogenic in the embyrofetal development study, which was expected based on its pharmacologic mechanism of action. Teratogenic effects included malformations of the head, mouth, face, and large blood vessels. In the pre- and postnatal study, sitaxentan administration was associated with reduced pup survival, large or abnormally shaped livers, and delays in markers of auditory and sexual development. Sitaxentan was detected in plasma of suckling pups receiving milk from females dosed with sitaxentan. These nonclinical study findings were reflected in the sitaxentan product label warnings.
Gateways to clinical trials.[Pubmed:20508873]
Methods Find Exp Clin Pharmacol. 2010 May;32(4):247-88.
O(6)-Benzylguanine; (-)-Gossypol; Abatacept, AC-2592, Adalimumab, AIDSVAX gp120 B/E, Alemtuzumab, Aliskiren fumarate, ALVAC E120TMG, Ambrisentan, Amlodipine, Anakinra, Aripiprazole, Armodafinil, Atomoxetine hydrochloride, Avotermin; Bevacizumab, BIBW-2992, Bortezomib, Bosentan, Botulinum toxin type B; Canakinumab, CAT-354, Ciclesonide, CMV gB vaccine, Corifollitropin alfa, Daptomycin, Darbepoetin alfa, Dasatinib, Denosumab; EndoTAG-1, Eplerenone, Esomeprazole sodium, Eszopiclone, Etoricoxib, Everolimus, Exenatide, Ezetimibe, Ezetimibe/simvastatin; F-50040, Fesoterodine fumavate, Fondaparinux sodium, Fulvestrant; Gabapentin enacarbil, Golimumab; Imatinib mesylate, Inhalable human insulin, Insulin glargine, Ivabradine hydrochloride; Lercanidipine hydrochloride/enalapril maleate, Levosimendan, Liposomal vincristine sulfate, Liraglutide; MDV-3100, Mometasone furoate/formoterol fumavate, Multiepitope CTL peptide vaccine, Mycophenolic acid sodium salt, Nabiximols, Natalizumab, Nesiritide; Obeticholic acid, Olmesartan medoxomil, Omalizumab, Omecamtiv mecarbil; Paclitaxel-eluting stent, Paliperidone, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b, Peginterferon alfa-2b/ ribavirin, Pemetrexed disodium, Polymyxin B nonapeptide, PORxin-302, Prasugrel, Pregabalin, Pridopidine; Ranelic acid distrontium salt, Rasagiline mesilate, rDEN4delta30-4995, Recombinant human relaxin H2, rhFSH, Rilonacept, Rolofylline, Rosiglitazone maleate/metformin hydrochloride, Rosuvastatin calcium, Rotigotine; Salcaprozic acid sodium salt, Sirolimus-eluting stent, Sitagliptin phosphate monohydrate, Sitaxentan sodium, Sorafenib, Sunitinib malate; Tadalafil, Tapentadol hydrochloride, Temsirolimus, Tenofovir, Tenofovir disoproxil fumarate, Teriparatide, Tiotropium bromide, Tocilizumab, Tolvaptan, Tozasertib, Treprostinil sodium; Ustekinumab; Vardenafil hydrochloride hydrate, Varenicline tartrate, Vatalanib succinate, Voriconazole, Vorinostat; Zotarolimus-eluting stent.
Gateways to clinical trials.[Pubmed:20383346]
Methods Find Exp Clin Pharmacol. 2010 Jan-Feb;32(1):47-86.
(-)-Epigallocatechin gallate, Abafungin, ACE-031, Adapalene/benzoyl peroxide, AE-37, Aflibercept, AGS-003, Albiglutide, Alemtuzumab, Aliskiren fumarate, ALT-801, AN-2728, Anacetrapib, API, Aprepitant, ARQ-197, Ascorbic acid, Atazanavir sulfate, ATN-224, AVI-4658, Azacitidine, Azelnidipine; Belinostat, Bevacizumab, BI-2536, Biphasic insulin aspart, Bortezomib, Bovine lactoferrin, Bryostatin 1, Budesonide/formoterol fumarate; cAC10, Canfosfamide hydrochloride, Cediranib, Clofarabine, Cocaine conjugate vaccine; Darbepoetin alfa, Dasatinib, Denosumab, Disomotide, Doripenem, Dovitinib Lactate, Dronedarone hydrochloride, Drospirenone/estradiol, Dutasteride; Ecogramostim, Entinostat, Enzastaurin hydrochloride, Erlotinib hydrochloride, Everolimus, Exenatide, Ezetimibe, Ezetimibe/simvastatin; Fampridine, Fenretinide LXS, FFR-factor VIIa, Fingolimod hydrochloride, Frovatriptan; Gefitinib, Gimatecan, GP-2/GM-CSF; Iloperidone, Imatinib mesylate, Indibulin, Ipilimumab, Ivabradine hydrochloride; Lactobacillus rhamnosus, Lapatinib ditosylate, LC-07, Lenalidomide, Linifanib, Liposomal doxorubicin, Liposomal vincristine, Litenimod, Lutein; M-118, MDX-1401, MEDI-528, Midostaurin, Miglustat, MK-0657; Natalizumab, Nesiritide, NGR-TNF, Niacin/simvastatin; Obatoclax mesylate, Olaparib, Omacetaxine mepesuccinate; Paclitaxel nanoparticles, Paclitaxel-eluting stent, Palonosetron hydrochloride, Pazopanib hydrochloride, Pegfilgrastim, Pemetrexed disodium, PER.C-flu, Perifosine, PF-02341066, Pimecrolimus, Pitrakinra, Plerixafor hydrochloride, Posaconazole; Rasburicase, Recombinant human relaxin H2, ReoT3D, Retaspimycin hydrochloride, Riferminogene pecaplasmid, Rindopepimut, Romiplostim, Ronacaleret hydrochloride, Rosuvastatin calcium, Rotigotine; Sagopilone, sALP-FcD10, SAR-245409, SCH-697243, Selumetinib, Sirolimus-eluting stent, SIR-Spheres, Sitagliptin phosphate monohydrate, Sitaxentan sodium, Sorafenib, Sunitinib malate; Tadalafil, Tandutinib, Tasimelteon, Temsirolimus, Teriparatide, Tiotropium bromide, TIV, Trabectedin, Tremelimumab, TRU-016; Vadimezan, Val8-GLP-1(7-37)OH, Vandetanib, Vernakalant hydrochloride, Voreloxin, Voriconazole, Vorinostat, Yttrium 90 (90Y) ibritumomab tiuxetan; Zeaxanthin, Ziprasidone hydrochloride, Zosuquidar trihydrochloride.
Gateways to clinical trials.[Pubmed:18560631]
Methods Find Exp Clin Pharmacol. 2008 Mar;30(2):149-71.
Gateways to Clinical Trials are a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs: 131-I-Chlorotoxin, 423557; Abatacept, Ad.Egr.TNF.11D, Adalimumab, AE-941, Ambrisentan, AMR-001, Anacetrapib, Anakinra, Aripiprazole, Atazanavir sulfate; BAY-639044, Bazedoxifene acetate, Belimumab, Bevacizumab, Bortezomib, Botulinum toxin type B, Brivaracetam, Bucindolol hydrochloride; Carfilzomib, Carisbamate, CCX-282, CD20Bi, Ceftobiprole, Certolizumab pegol, CF-101, Cinacalcet hydrochloride, Cypher; Darifenacin hydrobromide, Degarelix acetate, Denosumab, Desvenlafaxine succinate, Dexlansoprazole, Dexverapamil, Drotrecogin alfa (activated), Duloxetine hydrochloride, Dutasteride; Efalizumab, EPs-7630, Escitalopram oxalate, Etoricoxib; Fluticasone furoate, Fondaparinux sodium, Fospropofol disodium; Hexadecyloxypropyl-cidofovir, HIV gp120/NefTat/AS02A, HPV-6/11/16/18; INCB-18424, Incyclinide, Inhalable human insulin, Insulin detemir; KNS-760704, KW-0761; Lacosamide, Lenalidomide, Levetiracetam, Licofelone, Lidocaine/prilocaine; mAb 216, MEDI-528, Men ACWY, Meningococcal C-CRM197 vaccine, Methylnaltrexone bromide; Nemifitide ditriflutate, Nicotine conjugate vaccine, Nilotinib hydrochloride monohydrate; Octaparin; Parathyroid hormone (human recombinant), Pegaptanib octasodium, Pitrakinra, Prasterone, Pregabalin; Ranelic acid distrontium salt, Rasagiline mesilate, Retigabine, Rimonabant, RTS,S/AS02D; Sarcosine, Sitaxentan sodium, Solifenacin succinate, Sunitinib malate; Taranabant, Taxus, Teduglutide, Teriparatide, Ticagrelor, Travoprost, TRU-015; USlipristal acetate, Urocortin 2; Vardenafil hydrochloride hydrate; YM-155, Yttrium 90 (90Y) ibritumomab tiuxetan; Zanolimumab, Zoledronic acid monohydrate, Zotarolimus, Zotarolimus-eluting stent.


