Org 12962 hydrochlorideSelective 5-HT2C agonist CAS# 210821-63-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 210821-63-9 | SDF | Download SDF |
| PubChem ID | 9796407 | Appearance | Powder |
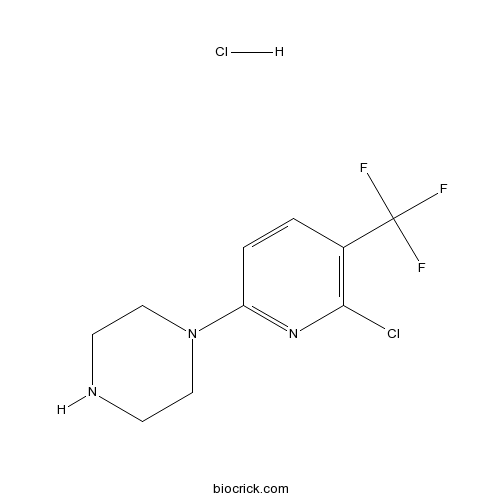
| Formula | C10H12Cl2F3N3 | M.Wt | 302.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 1-[6-chloro-5-(trifluoromethyl)pyridin-2-yl]piperazine;hydrochloride | ||
| SMILES | C1CN(CCN1)C2=NC(=C(C=C2)C(F)(F)F)Cl.Cl | ||
| Standard InChIKey | FVDILXYXZAAJOS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H11ClF3N3.ClH/c11-9-7(10(12,13)14)1-2-8(16-9)17-5-3-15-4-6-17;/h1-2,15H,3-6H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective 5-HT2C receptor agonist (pEC50 values are 7.01, 6.38 and 6.28 for 5-HT2C, 5-HT2A and 5-HT2B respectively). Displays antiaversive effects in a rat model of panic-like anxiety. |

Org 12962 hydrochloride Dilution Calculator

Org 12962 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3099 mL | 16.5497 mL | 33.0994 mL | 66.1989 mL | 82.7486 mL |
| 5 mM | 0.662 mL | 3.3099 mL | 6.6199 mL | 13.2398 mL | 16.5497 mL |
| 10 mM | 0.331 mL | 1.655 mL | 3.3099 mL | 6.6199 mL | 8.2749 mL |
| 50 mM | 0.0662 mL | 0.331 mL | 0.662 mL | 1.324 mL | 1.655 mL |
| 100 mM | 0.0331 mL | 0.1655 mL | 0.331 mL | 0.662 mL | 0.8275 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sakakin
Catalog No.:BCN4916
CAS No.:21082-33-7
- CP 471474
Catalog No.:BCC2373
CAS No.:210755-45-6
- 7,8-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8290
CAS No.:2107-77-9
- 5,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8284
CAS No.:2107-76-8
- PD 168568 dihydrochloride
Catalog No.:BCC7702
CAS No.:210688-56-5
- 6alpha-Hydroxylycopodine
Catalog No.:BCN7403
CAS No.:21061-92-7
- 1,11b-Dihydro-11b-hydroxymaackiain
Catalog No.:BCN3914
CAS No.:210537-05-6
- 1,11b-Dihydro-11b-hydroxymedicarpin
Catalog No.:BCN3913
CAS No.:210537-04-5
- Sitaxentan sodium
Catalog No.:BCC4495
CAS No.:210421-74-2
- Odoratin-7-O-beta-D-glucopyranoside
Catalog No.:BCN8089
CAS No.:210413-47-1
- Spiradine F
Catalog No.:BCN4915
CAS No.:21040-64-2
- Cinnamyl acetate
Catalog No.:BCN4914
CAS No.:21040-45-9
- W-84 dibromide
Catalog No.:BCC6682
CAS No.:21093-51-6
- CART (62-76) (rat, human)
Catalog No.:BCC6008
CAS No.:210978-19-1
- BMY 7378
Catalog No.:BCC5063
CAS No.:21102-95-4
- Mahanimbine
Catalog No.:BCN3174
CAS No.:21104-28-9
- SB 265610
Catalog No.:BCC5936
CAS No.:211096-49-0
- Marsformoxide B
Catalog No.:BCN6687
CAS No.:2111-46-8
- Sobetirome
Catalog No.:BCC1957
CAS No.:211110-63-3
- Rubranol
Catalog No.:BCN4917
CAS No.:211126-61-3
- 9,17-Octadecadiene-12,14-diyne-1,11,16-triol
Catalog No.:BCN1497
CAS No.:211238-60-7
- m-Chlorophenylbiguanide hydrochloride
Catalog No.:BCC6650
CAS No.:2113-05-5
- R18
Catalog No.:BCC2383
CAS No.:211364-78-2
- Astrocasine
Catalog No.:BCN2150
CAS No.:2114-92-3
Mianserin hydrochloride (Org GB 94) in the treatment of obsessional states.[Pubmed:881101]
J Int Med Res. 1977;5(4):289-91.
Nine patients with severe primary obsessional illness were treated with mianserin hydrochloride (Org GB 94), in a dose increasing to 20 mg three times daily, for 4 weeks. Six patients improved as assessed by a physician's global rating of improvement. The symptoms on a side-effects check list were less marked during treatment than they had been during the pre-treatment drug-free period.
Clinical experience with Org GB94 (mianserin hydrochloride), a new tetracyclic antidepressant.[Pubmed:669494]
Folia Psychiatr Neurol Jpn. 1978;32(2):171-83.
(1) Org GB94 (Mianserin hydrochloride), a new tetracyclic antidepressant, was administered to 21 patients with depression or with depressive states. (2) Remarkable antidepressive response was obtained with Org GB94 in moderate and mild forms of endogenous, involutional and senile depression. (3) The onset of action of Org GB94 was rapid. (4) Anticholinergic side effects were extremely rare and other untoward effects were mild in nature.
Mass fragmentographic assay of nanogram amounts of the antidepressant drug mianserin hydrochloride (Org GB 94) in human plasma.[Pubmed:863985]
J Chromatogr. 1977 May 1;143(3):289-97.
For the assay of the antidepressant compound mianserin hydrochloride (Org GB 94) in human plasma, a mass fragmentographic method, using the deuterated analogue as internal standard and a high-performance liquid chromatographie sample clean-up procedure has been developed. The assay specifications obtained are a lower limit for reliable measurements of 1 ng/ml, and accuracy of ca. 0.01 ng/ml, a precision of 6--7% and a capacity of about 60 samples per day. The applicability of the assay method is illustrated by measurements of single-dose and steady-state plasma levels in clinical experiments, demonstrating the possibility of monitoring plasma levels during at least 24 h after a single dose of 15 mg of Org GB 94. The mean steady-state plasma levels after a daily dose of 3 X 20 mg of Org GB 94 appeared to be remarkably constant with time: 38, 36 and 34 ng/ml after 2, 4, and 6 weeks of treatment of 18 depressed patients.
Antiarrhythmic, metabolic and hemodynamic effects of Org 6001 (3alpha-amino-5alpha-androstan-2beta-ol-17-one-hydrochloride) after coronary flow reduction in pigs.[Pubmed:633069]
J Pharmacol Exp Ther. 1978 Mar;204(3):634-44.
The antiarrhythmic activity of the aminosteroid Org 6001 was investigated in young pigs (20-28 kg). Ventricular arrhythmias were induced by restriction of the flow in the left anterior descending coronary artery (LAD) to 25% of its control value during a period of 30 minutes. Nine out of 30 control animals died in this period due to ventricular fibrillation. None of the 19 animals treated with Org 6001 (5-10 mg/kg) or the 12 animals treated with lidocaine (2.75-3.50 mg/kg) fibrillated. Moreover, the number of premature ventricular beats was greatly reduced in pretreated groups compared with the untreated group (P less than .001). The first derivative of left ventricular pressure decreased with 25% (P less than 0.001) after administration of Org 6001. However, during 30 minutes of LAD flow reduction to 25% of control, the adverse effects of Org 6001 were less than those of lidocaine. Myocardial lactate production indicated some delay in onset of ischemia. However, there was no indication that this beneficial effect was long-lasting. When after 30 minutes of LAD flow reduction to 25% of control, the LAD was completely occluded between its second and third branch, all untreated animals fibrillated within 120 minutes, whereas 4 of the 19 animals treated with Org 6001 and 3 of the 12 treated with lidocaine survived. It is concluded that Org 6001 has antiarrhythmic properties in the ischemic pig heart which compare favorably with those of lidocaine.
Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity.[Pubmed:15777190]
Curr Drug Targets. 2005 Mar;6(2):201-13.
The pivotal role of 5-HT in the control of appetite was formally proposed nearly 30 years ago. In particular endogenous hypothalamic 5-HT has been implicated in the processes of within meal satiation and the end state of post meal satiety. Of the numerous 5-HT receptor subtypes currently identified, 5-HT(1B) and 5-HT(2C) receptors are believed to mediate the 5-HT induced satiety. 5-HT drugs such as d-fenfluramine, selective serotoninergic reuptake inhibitor (SSRIs) and 5-HT(2C) receptor agonists have all been shown to significantly attenuate rodent body weight gain, an effect strongly associated with marked hypophagia. D-Fenfluramine, sibutramine, fluoxetine and the 5-HT(2C) receptor agonist mCPP have also all been shown to reduce caloric intake by modifying appetite in both lean and obese humans. Specifically, 5-HT drugs reduce appetite prior to and after the consumption of fixed caloric loads, and reduce pre meal appetite and caloric intake at ad libitum meals. Clinically significant weight loss over a year or more can be produced by both d-fenfluramine and sibutramine treatment, but apparently not by the SSRI fluoxetine. Treatment with the preferential 5-HT(2C) receptor agonist mCPP and the serotonin precursor 5-HTP has also been shown to produce weight loss in the obese. Issues around the actual and possible side effects of these compounds, and in the case of d-fenfluramine toxicity, have led to a search for drugs that act selectively on the CNS 5-HT receptors critical to the satiety response. Currently, a new generation of 5-HT(2C) selective agonists have been developed (including Ro 60-0175, Org 12962, VER-3323, BVT-933 and YM348) and at least one, ADP356, is currently undergoing clinical trials. Hopefully, such drugs will be as or even more effective at regulating appetite and controlling body weight, and will also be free of their predecessors' side effect.
Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells.[Pubmed:10498829]
Br J Pharmacol. 1999 Sep;128(1):13-20.
1. The goal of this study was to characterize the agonist pharmacology of human 5-HT2A, 5-HT2B and 5-HT2C (VSV) receptors expressed in CHO-K1 (Chinese hamster ovary) cells. 2. We used a fluorometric imaging plate reader (FLIPR) which allows rapid detection of rises in intracellular calcium levels upon the addition of agonists. 3. Stimulation of all three receptors by 5-HT caused a robust concentration dependent increase in intracellular calcium levels. No such effect was observed from non-transfected control CHO-K1 cells. 4. The rank order of potency of agonists at the different receptor subtypes varied. Tryptamines, BW-723C86, d-norfenfluramine, Ro 60-0175 and LSD exhibited the following rank order of potency; 5-HT2B>5-HT2C>5-HT2A. Piperazines such as m-Chlorophenylpiperazine (mCPP), ORG-12962, MK-212 and also ORG-37684 exhibited a rank order of potency of 5-HT2C>5-HT2B>5-HT2A. The phenylisopropylamines DOI and DOB had a rank order of 5-HT2A>5-HT2B>5-HT2C. 5. Many agonists tested had partial agonist actions when compared to 5-HT, and a wide range of relative efficacies were exhibited, which was cell line dependent. For example, mCPP had a relative efficacy of 65% at 5-HT2C receptors but <25% at either 5-HT2A or 5-HT2B receptors. 6. Interpretation of literature values of functional assays using different cell lines, different receptor expression levels and different receptor isoforms, is complex. Species differences and the previous use of antagonist radioligands to characterize agonist potency in binding assays emphasizes the importance of studying agonists in the same experiment using the same assay conditions and parental cell lines.
Antiaversive effects of 5HT2C receptor agonists and fluoxetine in a model of panic-like anxiety in rats.[Pubmed:9716307]
Eur Neuropsychopharmacol. 1998 Aug;8(3):161-8.
Dose-dependent increases in threshold for operant fear/escape responses of rats submitted to aversive stimulation of the dorsolateral periaqueductal gray (dPAG) were recorded following intraperitoneal injection of three chemically unrelated but selective 5HT2C receptor agonists (Ro 60-0175, Org 12962 and Ro 60-0332) and fluoxetine. The decreased sensitivity of rats to the acute panic-like aversion elicited by stimulation of this limbic periventricular region was detected at dosages devoid of impairing effects on the latencies needed for operant brain stimulation interruption. In this paradigm which has been validated as a simulation of acute anxiety with relevance to panic disorder, the selective activation of 5HT2C receptors by Ro 60-0175, Org 12962 or Ro 60-0332 induces effects analogous to those observed following benzodiazepine receptor activation by antipanic agents such as clonazepam or alprazolam or following non-selective and indirect 5HT receptor activation by fluoxetine. Potency and efficacy of 5HT2C receptor agonists were intermediate between those of clonazepam and fluoxetine, indicating authentic antiaversive properties and suggesting antipanic potential for these 5HT2C receptor agonists. In addition, these data suggest that the 5HT2C receptor subtype may play a major role in the therapeutic properties of selective serotonin reuptake inhibitors. It is also speculated that serotonin/benzodiazepine interactions existing in the brain may functionally involve the 5HT2C receptor subtypes and that the anxiogenic action reported under certain circumstances for 5HT mimetics are not mediated by 5HT2C receptor subtypes.


