Cinnamyl acetateCAS# 21040-45-9 |
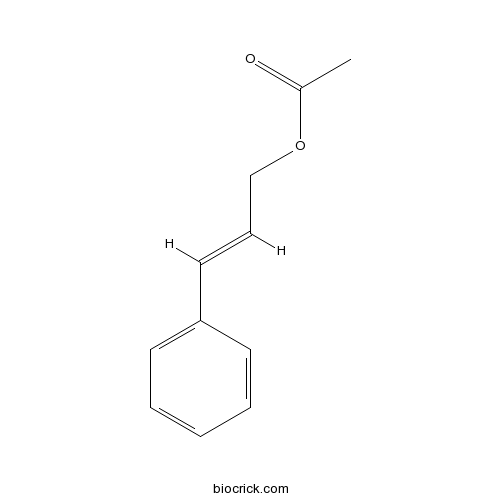
- Cinnamylacetate
Catalog No.:BCX0918
CAS No.:103-54-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21040-45-9 | SDF | Download SDF |
| PubChem ID | 5282110 | Appearance | Oil |
| Formula | C11H12O2 | M.Wt | 176.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | 103-54-8; 3-Phenyl-2-Propenyl Acetate; 3-Phenylallyl Acetate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(E)-3-phenylprop-2-enyl] acetate | ||
| SMILES | CC(=O)OCC=CC1=CC=CC=C1 | ||
| Standard InChIKey | WJSDHUCWMSHDCR-VMPITWQZSA-N | ||
| Standard InChI | InChI=1S/C11H12O2/c1-10(12)13-9-5-8-11-6-3-2-4-7-11/h2-8H,9H2,1H3/b8-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cinnamyl acetate is used as flavor and fragrance ingredient in food and cosmetic industries. |

Cinnamyl acetate Dilution Calculator

Cinnamyl acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6754 mL | 28.3768 mL | 56.7537 mL | 113.5074 mL | 141.8842 mL |
| 5 mM | 1.1351 mL | 5.6754 mL | 11.3507 mL | 22.7015 mL | 28.3768 mL |
| 10 mM | 0.5675 mL | 2.8377 mL | 5.6754 mL | 11.3507 mL | 14.1884 mL |
| 50 mM | 0.1135 mL | 0.5675 mL | 1.1351 mL | 2.2701 mL | 2.8377 mL |
| 100 mM | 0.0568 mL | 0.2838 mL | 0.5675 mL | 1.1351 mL | 1.4188 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- Ac-LEHD-AFC
Catalog No.:BCC2359
CAS No.:210345-03-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-IETD-FMK
Catalog No.:BCC5116
CAS No.:210344-98-2
- Z-DEVD-FMK
Catalog No.:BCC1137
CAS No.:210344-95-9
- Gallein
Catalog No.:BCC7563
CAS No.:2103-64-2
- 7-Hydroxycadalene
Catalog No.:BCN7501
CAS No.:2102-75-2
- 6-Formyl-1,2,9,10-tetramethoxy-6a,7-dehydroaporphine
Catalog No.:BCN6436
CAS No.:2101836-45-5
- Amarogentin
Catalog No.:BCN2661
CAS No.:21018-84-8
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- 5,8,9,10,14-Pentaacetoxy-3-benzoyloxy-15-hydroxypepluane
Catalog No.:BCN1498
CAS No.:210108-91-1
- Jatrophane VI
Catalog No.:BCN7659
CAS No.:210108-90-0
- Spiradine F
Catalog No.:BCN4915
CAS No.:21040-64-2
- Odoratin-7-O-beta-D-glucopyranoside
Catalog No.:BCN8089
CAS No.:210413-47-1
- Sitaxentan sodium
Catalog No.:BCC4495
CAS No.:210421-74-2
- 1,11b-Dihydro-11b-hydroxymedicarpin
Catalog No.:BCN3913
CAS No.:210537-04-5
- 1,11b-Dihydro-11b-hydroxymaackiain
Catalog No.:BCN3914
CAS No.:210537-05-6
- 6alpha-Hydroxylycopodine
Catalog No.:BCN7403
CAS No.:21061-92-7
- PD 168568 dihydrochloride
Catalog No.:BCC7702
CAS No.:210688-56-5
- 5,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8284
CAS No.:2107-76-8
- 7,8-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8290
CAS No.:2107-77-9
- CP 471474
Catalog No.:BCC2373
CAS No.:210755-45-6
- Sakakin
Catalog No.:BCN4916
CAS No.:21082-33-7
- Org 12962 hydrochloride
Catalog No.:BCC7718
CAS No.:210821-63-9
Commercial Origanum compactum Benth. and Cinnamomum zeylanicum Blume essential oils against natural mycoflora in Valencia rice.[Pubmed:25612221]
Nat Prod Res. 2015;29(23):2215-8.
Chemical composition of commercial Origanum compactum and Cinnamomum zeylanicum essential oils and the antifungal activity against pathogenic fungi isolated from Mediterranean rice grains have been investigated. Sixty-one compounds accounting for more than 99.5% of the total essential oil were identified by using gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). Carvacrol (43.26%), thymol (21.64%) and their biogenetic precursors p-cymene (13.95%) and gamma-terpinene (11.28%) were the main compounds in oregano essential oil, while the phenylpropanoids, eugenol (62.75%), eugenol acetate (16.36%) and (E)-Cinnamyl acetate (6.65%) were found in cinnamon essential oil. Both essential oils at 300 mug/mL showed antifungal activity against all tested strains. O. compactum essential oil showed the best antifungal activity towards Fusarium species and Bipolaris oryzae with a total inhibition of the mycelial growth. In inoculated rice grains at lower doses (100 and 200 mug/mL) significantly reduced the fungal infection, so O. compactum essential oil could be used as ecofriendly preservative for field and stored Valencia rice.
Catalytic Nucleophilic Allylation Driven by the Water-Gas Shift Reaction.[Pubmed:29220183]
J Org Chem. 2018 Jan 5;83(1):23-48.
The ruthenium-catalyzed allylation of aldehydes with allylic pro-nucleophiles has been demonstrated to be an efficient means to form carbon-carbon bonds under mild conditions. The evolution of this reaction from the initial serendipitous discovery to its general synthetic scope is detailed, highlighting the roles of water, CO, and amine in the generation of a more complete catalytic cycle. The use of unsymmetrical allylic pro-nucleophiles was shown to give preferential product formation through the modulation of reaction conditions. Both (E)-Cinnamyl acetate and vinyl oxirane were efficiently used to form the anti-branched products (up to >20:1 anti/syn) and E-linear products (up to >20:1 E/Z) in high selectivity with aromatic, alpha,beta-unsaturated, and aliphatic aldehydes, respectively. Attempts to render the reaction enantioselective are highlighted and include enantioenrichment of up to 75:25 for benzaldehyde.
A novel step towards immobilization of biocatalyst using agro waste and its application for ester synthesis.[Pubmed:29733931]
Int J Biol Macromol. 2018 Oct 1;117:366-376.
This work explains the utilization of agro-waste (coconut and peanut shell) to produce mesoporous activated carbon which further utilized as a support material for lipase immobilization (Candida antarctica B, CALB). Various parameters affecting the binding of enzyme to activated carbon with high surface area (1603m(2)g(-1)) were optimized. Maximum 200mugg(-1) CALB has been loaded at 40 degrees C and pH6.8 in 12h by using glutaraldehyde as a cross-linker. The operational parameters such as pH (5.8-8.8) and temperature (30-70 degrees C) were optimized for free and immobilized form of lipase. In thermal stability (50-70 degrees C) study, immobilization of enzyme showed 2.35 folds increased half-life with respect to free enzyme. The samples, before and after immobilization, were characterized by specific surface area, FT-IR, SEM, XRD. This immobilized lipase was successfully used for the synthesis of Cinnamyl acetate by transesterification reaction producing 94% conversion in 60min. Catalytic efficiency (58+/-1.08) was seen to be retained for more than five consecutive cycles of chemical reaction for repeated applications. Sequential results towards activity retention were obtained upto 30days of storability study. In the context, this process constitutes a clean route for the development of sustainable biocatalysts from agro waste, capable of applications in various areas.
Whole-Cell Biocatalytic Synthesis of Cinnamyl Acetate with a Novel Esterase from the DNA Library of Acinetobacter hemolyticus.[Pubmed:28220703]
J Agric Food Chem. 2017 Mar 15;65(10):2120-2128.
Cinnamyl acetate has a wide application in the flavor and fragrance industry because of its sweet, balsamic, and floral odor. Up to now, lipases have been mainly used in enzyme-mediated synthesis of Cinnamyl acetate, whereas esterases are used in only a few cases. Moreover, the use of purified enzymes is often a disadvantage, which leads to increases of the production costs. In this paper, a genomic DNA library of Acinetobacter hemolyticus was constructed, and a novel esterase (EstK1) was identified. After expression in Escherichia coli, the whole-cell catalyst of EstK1 displayed high transesterification activity to produce Cinnamyl acetate in nonaqueous systems. Furthermore, under optimal conditions (vinyl acetate as acyl donor, isooctane as solvent, molar ratio 1:4, temperature 40 degrees C), the conversion ratio of cinnamyl alcohol could be up to 94.1% at 1 h, and it reached an even higher level (97.1%) at 2 h.
Controlled delivery of a new broad spectrum antibacterial agent against colitis: In vitro and in vivo performance.[Pubmed:26209123]
Eur J Pharm Biopharm. 2015 Oct;96:152-61.
Coated pellets and mini-tablets were prepared containing a new broad spectrum antibacterial agent: CIN-102, a well-defined, synergistic blend of trans-cinnamaldehyde, trans-2-methoxycinnamaldehyde, Cinnamyl acetate, linalool, beta-caryophyllene, cineol and benzyl benzoate. The aim was to provide a new treatment method for colitis, especially for Inflammatory Bowel Disease (IBD) patients. Since the simple oral gavage of CIN-102 was not able to reduce the pathogenic bacteria involved in colitis (rat model), the drug was incorporated into multiparticulates. The idea was to minimize undesired drug release in the upper gastrointestinal tract and to control CIN-102 release in the colon, in order to optimize the resulting antibiotic concentration at the site of action. A particular challenge was the fact that CIN-102 is a volatile hydrophobic liquid. Pellet cores were prepared by extrusion-spheronization and coated with polymer blends, which are sensitive to colonic bacterial enzymes. Mini-tablets were prepared by direct compression. The release of the main compound of CIN-102 (cinnamaldehyde, 86.7% w/w) was monitored in vitro. Optimized coated pellets and mini-tablets were also tested in vivo: in seven-week-old, male mice suffering from dextran sodium sulfate induced colitis. Importantly, both types of multiparticulates were able: (i) to significantly reduce the number of luminal and mucosal enterobacteria in the mice (the levels of which are increased in the disease state), and (ii) to improve the clinical course of the intestinal inflammation (decrease in the percentages of mice with bloody stools and diarrhea). Thus, the proposed coated pellets and matrix mini-tablets allowing for controlled CIN-102 release show a promising potential for new treatment methods of colitis.
Fragrance material review on cinnamyl acetate.[Pubmed:18031892]
Food Chem Toxicol. 2007;45 Suppl 1:S53-7.
A toxicologic and dermatologic review of Cinnamyl acetate when used as a fragrance ingredient is presented.
Pd-Catalyzed Direct C-H Alkenylation and Allylation of Azine N-Oxides.[Pubmed:29629776]
Org Lett. 2018 Apr 20;20(8):2346-2350.
A Pd-catalyzed direct C2-alkenylation of azine N-oxides with allyl acetate is disclosed. The products are formed through an allylation/isomerization cascade process. The use of a tri- tert-butylphosphonium salt as the ligand precursor and KF is mandatory for optimal yields. When Cinnamyl acetate is used, the same catalytic system promotes C2-cinnamylation of the azine N-oxide without subsequent isomerization. A mechanism is proposed on the basis of experimental studies and DFT calculations.
Ultrasound assisted lipase catalyzed synthesis of cinnamyl acetate via transesterification reaction in a solvent free medium.[Pubmed:26186841]
Ultrason Sonochem. 2015 Nov;27:241-246.
Cinnamyl acetate is known for its use as flavor and fragrance material in different industries such as food, pharmaceutical, cosmetic etc. This work focuses on ultrasound assisted lipase (Novozym 435) catalyzed synthesis of Cinnamyl acetate via transesterification of cinnamyl alcohol and vinyl acetate in non-aqueous, solvent free system. Optimization of various parameters shows that a higher yield of 99.99% can be obtained at cinnamyl alcohol to vinyl acetate ratio of 1:2 with 0.2% of catalyst, at 40 degrees C and 150 rpm, with lower ultrasound power input of 50 W (Ultrasound intensity 0.81 W/cm(2)), at 25 kHz frequency, 50% duty cycle. Further, the time required for the maximum conversion is reduced to 20 min as compared to 60 min of conventional process. Similarly, the enzyme can be successfully reused seven times without loss of enzyme activity. Thus, ultrasound helps to enhance the enzyme catalyzed synthesis of flavors.


