Z-VAD-FMKCell-permeable, irreversible pan-caspase inhibitor CAS# 187389-52-2 |
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 187389-52-2 | SDF | Download SDF |
| PubChem ID | 5497174 | Appearance | Powder |
| Formula | C22H30FN3O7 | M.Wt | 467.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Z-Val-Ala-Asp(OMe)-FMK | ||
| Solubility | DMSO : 100 mg/mL (213.91 mM; Need ultrasonic) | ||
| Sequence | VAD (Modifications: Val-1 = Z-Val, Asp-3 = (OMe)-fluoromethylketone) | ||
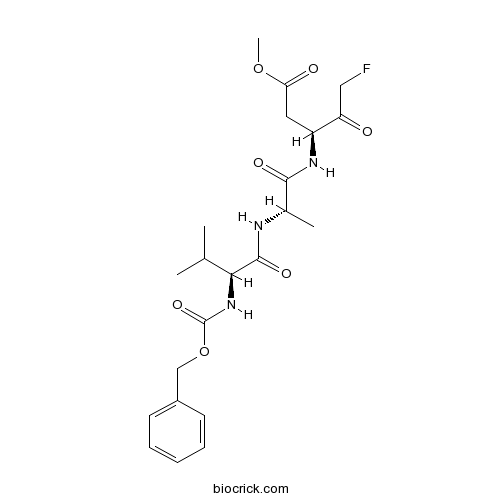
| Chemical Name | methyl (3S)-5-fluoro-3-[[(2S)-2-[[(2S)-3-methyl-2-(phenylmethoxycarbonylamino)butanoyl]amino]propanoyl]amino]-4-oxopentanoate | ||
| SMILES | CC(C)C(C(=O)NC(C)C(=O)NC(CC(=O)OC)C(=O)CF)NC(=O)OCC1=CC=CC=C1 | ||
| Standard InChIKey | MIFGOLAMNLSLGH-QOKNQOGYSA-N | ||
| Standard InChI | InChI=1S/C22H30FN3O7/c1-13(2)19(26-22(31)33-12-15-8-6-5-7-9-15)21(30)24-14(3)20(29)25-16(17(27)11-23)10-18(28)32-4/h5-9,13-14,16,19H,10-12H2,1-4H3,(H,24,30)(H,25,29)(H,26,31)/t14-,16-,19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Z-VAD-FMK is a cell-permeable, pan-caspase inhibitor. |

Z-VAD-FMK Dilution Calculator

Z-VAD-FMK Molarity Calculator
Abstract
The caspase inhibitor Z-VAD-FMK exhibits immunosuppressive and anti-proliferative activities in T cell without inhibiting the activity of caspases 8 and 3, where it inhibits NF-KB, the expression of CD25 and FasL-induced apoptosis.
Abstract
The effects of Z-VAD-FMK, a caspase inhibitor, on T lymphocytes and the elicitation of murine ACD were investigated.
Abstract
Intracerebroventricular administration of Z-VAD-FMK, a caspase inhibitor restricted by blood-brain barrier, to post-radiation rats resulted in reduced numbers of TUNEL-positive cells in the hypoglossal nucleus, suppressed expression and activation of caspases 3/8/9 and decrease appearance of cytochrome c in the cytosolic fraction.
Abstract
Z-VAD-FMK is a caspase inhibitor that inhibits mitochondria-related apoptotic effects induced by CIN.
Abstract
Early direct infusion of Z-VAD-FMK, a caspase inhibitor, into cochlear leads to accelerated hearing recovery and reduced hair cell loss in pigs suffering gunshot noise-induced trauma.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-VAD-FMK, an inhibitor of ICE-like proteases, inhibits apoptosis in THP.1 cells induced by diverse stimuli1 and Fas antigen-induced apoptosis in Jurkat T-cells2. It inhibits apoptosis by blocking the activation of proCPP32 into its active form, rather than by preventing the proteolytic action of CPP32 directly.
Z- VAD-FMK inhibits the formation of large kilobasepair fragments of DNA induced by diverse stimuli. Z-VAD-FMK had little or no effect on STS-induced necrotic cell death suggesting that the ICE-like protease activity was not involved in necrosis3.
Z-VAD-FMK almost completely inhibited the formation of large kilobasepair induced by all four stimuli. Similarly Z-VAD-FMK almost completely inhibited the enhanced formation of large kilobasepair fragments induced by thapsigargin or cycloheximide in the presence of TLCK, in good agreement with its ability to inhibit apoptosis induced by these treatments. These stimuli also induced internucleosomal cleavage of DNA, which was inhibited by Z-VAD-FMK. These results suggested that an ICE-like protease(s) acts at a stage prior to the formation of large kilobasepair fragments of DNA3.
References:
1. Darmon, A.J., Ehrman, N., Caputo, A., Fujinaga, J. and Bleackley, R.C. (1994) J. Biol. Chem. 269, 32043-32046.
2. Chow, S. C., Weis M., Kass, G. E. N., Holmstrom, T. H., Eriksson, J. E. and Orrenius S. (1995) FEBS Lett. 364, 134±138
3. H. Zhu et al./FEBS Letters 374 (1995) 303-308
- Kimcuongin
Catalog No.:BCN7472
CAS No.:1872403-23-0
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Oseltamivir acid
Catalog No.:BCC1826
CAS No.:187227-45-8
- Cyanidin-3-O-rutinoside chloride
Catalog No.:BCN3114
CAS No.:18719-76-1
- Luliconazole
Catalog No.:BCC1711
CAS No.:187164-19-8
- Clauszoline M
Catalog No.:BCN4683
CAS No.:187110-72-1
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- N,N'-Bis(2-hydroxyethyl)oxamide
Catalog No.:BCC9061
CAS No.:1871-89-2
- Sinapine
Catalog No.:BCN1815
CAS No.:18696-26-9
- Pafuramidine
Catalog No.:BCC1832
CAS No.:186953-56-0
- 2B-(SP)
Catalog No.:BCC5817
CAS No.:186901-17-7
- Moxifloxacin HCl
Catalog No.:BCC2507
CAS No.:186826-86-8
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- ER 50891
Catalog No.:BCC7783
CAS No.:187400-85-7
- Methylisopelletierine
Catalog No.:BCN1160
CAS No.:18747-42-7
- Sitoindoside I
Catalog No.:BCN1161
CAS No.:18749-71-8
- N-Aminophthalimide
Catalog No.:BCC9085
CAS No.:1875-48-5
- MaxiPost
Catalog No.:BCC7984
CAS No.:187523-35-9
- Ethyl rutinoside
Catalog No.:BCC8976
CAS No.:187539-57-7
- Fmoc-D-Arg(Pbf)-OH
Catalog No.:BCC3077
CAS No.:187618-60-6
- Tataramide B
Catalog No.:BCN3897
CAS No.:187655-56-7
- PNU 109291
Catalog No.:BCC7408
CAS No.:187665-60-7
- PNU 142633
Catalog No.:BCC7222
CAS No.:187665-65-2
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
In vitro evaluation of the anti-apoptotic drug Z-VAD-FMK on human ovarian granulosa cell lines for further use in ovarian tissue transplantation.[Pubmed:26169075]
J Assist Reprod Genet. 2015 Oct;32(10):1551-9.
PURPOSE: Because ovarian granulosa cells are essential for oocyte survival, we examined three human granulosa cell lines as models to evaluate the ability of the pan-caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (Z-VAD-FMK) to prevent primordial follicle loss after ovarian tissue transplantation. METHODS: To validate the efficacy of Z-VAD-FMK, three human granulosa cell lines (GC1a, HGL5, COV434) were treated for 48 h with etoposide (50 mug/ml) and/or Z-VAD-FMK (50 muM) under normoxic conditions. To mimic the ischemic phase that occurs after ovarian fragment transplantation, cells were cultured without serum under hypoxia (1 % O(2)) and treated with Z-VAD-FMK. The metabolic activity of the cells was evaluated by WST-1 assay. Cell viability was determined by FACS analyses. The expression of apoptosis-related molecules was assessed by RT-qPCR and Western blot analyses. RESULTS: Our assessment of metabolic activity and FACS analyses in the normoxic experiments indicate that Z-VAD-FMK protects granulosa cells from etoposide-induced cell death. When cells are exposed to hypoxia and serum starvation, their metabolic activity is reduced. However, Z-VAD-FMK does not provide a protective effect. In the hypoxic experiments, the number of viable cells was not modulated, and we did not observe any modifications in the expressions of apoptosis-related molecules (p53, Bax, Bcl-xl, and poly (ADP-ribose) polymerase (PARP)). CONCLUSION: The death of granulosa cell lines was not induced in our ischemic model. Therefore, a protective effect of Z-VAD-FMK in vitro for further use in ovarian tissue transplantation could not be directly confirmed. It will be of interest to potentially use Z-VAD-FMK in vivo in xenograft models.
Combined treatment of carfilzomib and z-VAD-fmk inhibits skeletal proteolysis and apoptosis and ameliorates cancer cachexia.[Pubmed:25737433]
Med Oncol. 2015 Apr;32(4):100.
The purpose of the study was to evaluate the therapeutic benefit of treatments with carfilzomib (CFZ) and Z-VAD-FMK in a mouse model of cancer-induced cachexia. The model of cancer-associated cachexia was generated by injecting murine C26 adenocarcinoma cells into BALB/C mice. CFZ and Z-VAD-FMK were administered individually or in combination at 5 and 12 days after inoculation. Changes in body weight, gastrocnemius muscle mass, tumor burden, spontaneous activity, survival, and metabolic profiles were noted. Also evaluated were the circulatory levels of renin and angiotensin II, and levels of apoptotic, proteolytic, and renin-angiotensin system-associated markers and transcription factor 2 (ATF2) in gastrocnemius muscle. The CFZ and Z-VAD-FMK treatments were associated with less muscle wasting, reduced tumor burden, modulated metabolism, higher levels of glucose, albumin, and total proteins, and lower levels of triglyceride fatty acids, more spontaneous physical activity, and longer survival in C26-inoculated mice compared with PBS-treated cachectic mice. CFZ and Z-VAD-FMK treatments resulted in higher levels of caspase-3 and BAX and lower level of BCL-XL in gastrocnemius muscles and altered the level of proteins in the renin-angiotensin system. The combined treatment administered 5 days after C26 inoculation was more effective than other regimens. Combined treatment with CFZ and Z-VAD-FMK early in the development of cachexia was associated with signs of less proteolysis and apoptosis and less severe cachexia in a mouse model of cancer-induced cachexia.
Positive effect of apoptotic inhibitor z-vad-fmk on vitrified-thawed porcine mii stage oocytes.[Pubmed:27393955]
Cryo Letters. 2016 May-Jun;37(3):188-95.
BACKGROUND: The developmental potential of vitrified porcine oocytes is very lower, and apoptosis is considered as one of the key factors involved. OBJECTIVE: To investigate the effects of apoptotic inhibitor Z-VAD-FMK addition into the incubation medium after warming on apoptosis and developmental ability of vitrified porcine MII-stage oocytes. MATERIALS AND METHODS: The activities of several caspases, mitochondrial membrane potential (DeltaPsim) and early apoptotic levels were measured. Parthenogenetic developmental ability and relative expression levels of apoptosis related genes were also detected. RESULTS: Caspase activity and early apoptotic level of the Z-VAD-FMK group were significantly lower than those of the group without Z-VAD-FMK addition, but were much higher than those of fresh group (P < 0.05). The DeltaPsim of Z-VAD-FMK group was 1.19, higher than the vitrified group (0.91) and lower than the fresh group (1.33). The cleavage rate and blastocyst rate after parthenogenetic activation in the Z-VAD-FMK group were much higher than those in the vitrified group, and much lower than those in the fresh group (P < 0.05). Vitrified porcine oocytes exhibited increased expression of pro-apoptotic genes (caspase 3, 8, 9, TNF-alpha) and decreased genes expression levels of anti-apoptotic genes (Bcl-2, CuZnSOD), and the Z-VAD-FMK addition in incubaiton medium significantly decreased the transcripts levels of caspase 3,8,9, Bax, TNF-alpha and increased Bcl-2 and CuZnSOD genes expression. CONCLUSION: The addition of apoptotic inhibitor Z-VAD-FMK into the incubation medium after warming improved the in vitro developmental ability of vitrified porcine oocytes by increasing mitochondrial function, reducing apoptotic level and changing apoptosis-elated gene expression.
Processing/activation of caspases, -3 and -7 and -8 but not caspase-2, in the induction of apoptosis in B-chronic lymphocytic leukemia cells.[Pubmed:9766499]
Leukemia. 1998 Oct;12(10):1553-60.
Chlorambucil and prednisolone, two commonly used drugs in the treatment of chronic lymphocytic leukemia (CLL), induce apoptosis in CLL cells. We have investigated the involvement in this apoptotic cell death of caspases, which cleave critical cellular substrates thereby acting as the executioners of the apoptotic process. Induction of spontaneous or chlorambucil/prednisolone-induced apoptosis of freshly isolated B-CLL cells in culture resulted in the activation of the 'effector' caspases, -3 and -7, but generally not of caspase-2. Activation of caspases-3 and -7 was accompanied by the proteolysis of the DNA repair enzyme, poly (ADP-ribose) polymerase. Induction of apoptosis was also accompanied by the processing of caspase-8, the extent of which varied between patients. Induction of apoptosis and processing of all the caspases was inhibited by the cell permeable caspase inhibitor, benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethyl ketone (Z-VAD.fmk). Our results demonstrate a key role for the activation and processing of caspases in the execution phase of apoptosis in CLL cells. Apoptosis of CLL cells resulted in the selective activation of some but not all caspases. Our results suggest that the dysregulation of apoptosis observed in CLL may be due to the signalling leading to the activation of caspases rather than a deletion of pro-caspases. High levels of caspase-8 in CLL cells in conjunction with low levels of CD95 receptor may offer new therapeutic opportunities for the treatment of CLL.
ICE-protease inhibitors block murine liver injury and apoptosis caused by CD95 or by TNF-alpha.[Pubmed:9093874]
Immunol Lett. 1997 Jan;55(1):5-10.
The two apoptosis receptors of mammalian cells, i.e. the 55 kDa TNF receptor (TNF-R1) and CD95 (Fas/APO1) are activated independently of each other, however, their signaling involves a variety of ICE-related proteases [I]. We used a cell-permeable inhibitor of ICE-like protease activity to examine in vivo whether post-receptor signaling of TNF and CD95 are fully independent processes. Mice pretreated with the inhibitor, Z-VAD-fluoromethylketone (FMK) were dose-dependently protected from liver injury caused by CD95 activation as determined by plasma alanine aminotransferase and also from hepatocyte apoptosis assessed by DNA fragmentation (ID50 = 0.1 mg/kg). A dose of 10 mg/kg protected mice also from liver injury induced by TNF-alpha. Similar results were found when apoptosis was initiated via TNF-alpha or via CD95 in primary murine hepatocytes (IC50 = 1.5 nM) or in various human cell lines. In addition to prevention, an arrest of cell death by Z-VAD-FMK was demonstrated in vivo and in vitro after stimulation of apoptosis receptors. These findings show in vitro and in vivo in mammals that CD95 and the TNF-alpha receptor share a distal proteolytic apoptosis signal.
Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32.[Pubmed:8670109]
Biochem J. 1996 Apr 1;315 ( Pt 1):21-4.
Interleukin-1 beta converting enzyme (ICE)-like proteases, which are synthesized as inactive precursors, play a key role in the induction of apoptosis. We now demonstrate that benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK), an ICE-like protease inhibitor, inhibits apoptosis by preventing the processing of CPP32 to its active form. These results suggest that novel inhibitors of apoptosis can be developed which prevent processing of proforms of ICE-like proteases.


