PafuramidineProdrug of furamidine CAS# 186953-56-0 |
- Raltitrexed
Catalog No.:BCC4457
CAS No.:112887-68-0
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 186953-56-0 | SDF | Download SDF |
| PubChem ID | 5480200 | Appearance | Powder |
| Formula | C20H20N4O3 | M.Wt | 364.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DB289 | ||
| Solubility | DMSO : 33.33 mg/mL (91.47 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
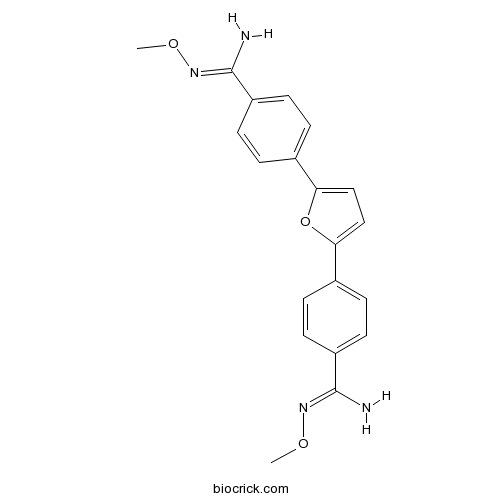
| Chemical Name | N'-methoxy-4-[5-[4-[(Z)-N'-methoxycarbamimidoyl]phenyl]furan-2-yl]benzenecarboximidamide | ||
| SMILES | CON=C(C1=CC=C(C=C1)C2=CC=C(O2)C3=CC=C(C=C3)C(=NOC)N)N | ||
| Standard InChIKey | UKOQVLAXCBRRGH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H20N4O3/c1-25-23-19(21)15-7-3-13(4-8-15)17-11-12-18(27-17)14-5-9-16(10-6-14)20(22)24-26-2/h3-12H,1-2H3,(H2,21,23)(H2,22,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pafuramidine (DB289) is an orally bioavailable prodrug of furamidine, which has clinical activity against Pneumocystis pneumonia.
IC50 Value: 4.5 nM (In vitro inhibitory activity against Trypanosoma brucei rhodesiense) [4]
Target: Antiparasitic
DB289 (pafuramidine maleate; 2,5-bis[4-(N-methoxyamidino)phenyl]furan monomaleate) is a prodrug of DB75 (furamidine dihydrochloride; 2,5-bis(4-guanylphenyl)furan dihydrochloride), an aromatic dication related to pentamidine that has demonstrated good efficacy against African trypanosomiasis, Pneumocystis carinii pneumonia, and malaria, but lacks adequate oral availability.
in vitro: The results of this investigation suggest that DB75 inhibits mitochondrial function. Yeast cells relying upon mitochondrial metabolism for energy production are especially sensitive to DB75 [1].
in vivo: Clearance of DB289 approximated the liver plasma flow and its large volume of distribution was consistent with extensive tissue binding. Plasma protein binding of DB289 was 97 to 99% in four animal species and humans, but that of DB75 was noticeably less and more species- and concentration-dependent [2]. Despite excellent oral activity against early-stage sleeping sickness, oral administration of DB289 exhibited limited efficacy in mouse models of late-stage disease [3].
Clinical trial: DB289, a novel orally active prodrug of DB75, is undergoing phase IIb clinical trials for early-stage human African trypanosomiasis, Pneumocystis jiroveci carinii pneumonia, and malaria [1]. References: | |||||

Pafuramidine Dilution Calculator

Pafuramidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7442 mL | 13.7212 mL | 27.4424 mL | 54.8847 mL | 68.6059 mL |
| 5 mM | 0.5488 mL | 2.7442 mL | 5.4885 mL | 10.9769 mL | 13.7212 mL |
| 10 mM | 0.2744 mL | 1.3721 mL | 2.7442 mL | 5.4885 mL | 6.8606 mL |
| 50 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0977 mL | 1.3721 mL |
| 100 mM | 0.0274 mL | 0.1372 mL | 0.2744 mL | 0.5488 mL | 0.6861 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 4.5 nM(In vitro inhibitory activity against Trypanosoma brucei rhodesiense) [4] DB289 (pafuramidine maleate; 2,5-bis[4-(N-methoxyamidino)phenyl]furan monomaleate) is a prodrug of DB75 (furamidine dihydrochloride; 2,5-bis(4-guanylphenyl)furan dihydrochloride), an aromatic dication related to pentamidine that has demonstrated good efficacy against African trypanosomiasis, Pneumocystis carinii pneumonia, and malaria, but lacks adequate oral availability. in vitro: The results of this investigation suggest that DB75 inhibits mitochondrial function. Yeast cells relying upon mitochondrial metabolism for energy production are especially sensitive to DB75 [1]. in vivo: Clearance of DB289 approximated the liver plasma flow and its large volume of distribution was consistent with extensive tissue binding. Plasma protein binding of DB289 was 97 to 99% in four animal species and humans, but that of DB75 was noticeably less and more species- and concentration-dependent [2]. Despite excellent oral activity against early-stage sleeping sickness, oral administration of DB289 exhibited limited efficacy in mouse models of late-stage disease [3]. Clinical trial: DB289, a novel orally active prodrug of DB75, is undergoing phase IIb clinical trials for early-stage human African trypanosomiasis, Pneumocystis jiroveci carinii pneumonia, and malaria [1].
- 2B-(SP)
Catalog No.:BCC5817
CAS No.:186901-17-7
- Moxifloxacin HCl
Catalog No.:BCC2507
CAS No.:186826-86-8
- ML 10302 hydrochloride
Catalog No.:BCC7695
CAS No.:186826-17-5
- Ginsenoside Rg5
Catalog No.:BCN3551
CAS No.:186763-78-0
- Alisol A 24-acetate
Catalog No.:BCN2344
CAS No.:18674-16-3
- N6-methyladenosine (m6A)
Catalog No.:BCC6495
CAS No.:1867-73-8
- Ketamine hydrochloride
Catalog No.:BCC5982
CAS No.:1867-66-9
- H-D-Tyr(tBu)-OH
Catalog No.:BCC3137
CAS No.:186698-58-8
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- 2-NBDG
Catalog No.:BCC6530
CAS No.:186689-07-6
- 4-Methylcinnamic acid
Catalog No.:BCN5034
CAS No.:1866-39-3
- Allyl cinnamate
Catalog No.:BCC8812
CAS No.:1866-31-5
- Sinapine
Catalog No.:BCN1815
CAS No.:18696-26-9
- N,N'-Bis(2-hydroxyethyl)oxamide
Catalog No.:BCC9061
CAS No.:1871-89-2
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- Clauszoline M
Catalog No.:BCN4683
CAS No.:187110-72-1
- Luliconazole
Catalog No.:BCC1711
CAS No.:187164-19-8
- Cyanidin-3-O-rutinoside chloride
Catalog No.:BCN3114
CAS No.:18719-76-1
- Oseltamivir acid
Catalog No.:BCC1826
CAS No.:187227-45-8
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Kimcuongin
Catalog No.:BCN7472
CAS No.:1872403-23-0
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- ER 50891
Catalog No.:BCC7783
CAS No.:187400-85-7
Pharmacology of DB844, an orally active aza analogue of pafuramidine, in a monkey model of second stage human African trypanosomiasis.[Pubmed:22848769]
PLoS Negl Trop Dis. 2012;6(7):e1734.
Novel drugs to treat human African trypanosomiasis (HAT) are still urgently needed despite the recent addition of nifurtimox-eflornithine combination therapy (NECT) to WHO Model Lists of Essential Medicines against second stage HAT, where parasites have invaded the central nervous system (CNS). The pharmacology of a potential orally available lead compound, N-methoxy-6-{5-[4-(N-methoxyamidino) phenyl]-furan-2-yl}-nicotinamidine (DB844), was evaluated in a vervet monkey model of second stage HAT, following promising results in mice. DB844 was administered orally to vervet monkeys, beginning 28 days post infection (DPI) with Trypanosoma brucei rhodesiense KETRI 2537. DB844 was absorbed and converted to the active metabolite 6-[5-(4-phenylamidinophenyl)-furanyl-2-yl]-nicotinamide (DB820), exhibiting plasma C(max) values of 430 and 190 nM for DB844 and DB820, respectively, after the 14th dose at 6 mg/kg qd. A 100-fold reduction in blood trypanosome counts was observed within 24 h of the third dose and, at the end of treatment evaluation performed four days post the last drug dose, trypanosomes were not detected in the blood or cerebrospinal fluid of any monkey. However, some animals relapsed during the 300 days of post treatment monitoring, resulting in a cure rate of 3/8 (37.5%) and 3/7 (42.9%) for the 5 mg/kgx10 days and the 6 mg/kgx14 days dose regimens respectively. These DB844 efficacy data were an improvement compared with pentamidine and Pafuramidine both of which were previously shown to be non-curative in this model of CNS stage HAT. These data show that synthesis of novel diamidines with improved activity against CNS-stage HAT was possible.
Efficacy and Safety of Pafuramidine versus Pentamidine Maleate for Treatment of First Stage Sleeping Sickness in a Randomized, Comparator-Controlled, International Phase 3 Clinical Trial.[Pubmed:26882015]
PLoS Negl Trop Dis. 2016 Feb 16;10(2):e0004363.
BACKGROUND: Sleeping sickness (human African trypanosomiasis [HAT]) is a neglected tropical disease with limited treatment options that currently require parenteral administration. In previous studies, orally administered Pafuramidine was well tolerated in healthy patients (for up to 21 days) and stage 1 HAT patients (for up to 10 days), and demonstrated efficacy comparable to pentamidine. METHODS: This was a Phase 3, multi-center, randomized, open-label, parallel-group, active control study where 273 male and female patients with first stage Trypanosoma brucei gambiense HAT were treated at six sites: one trypanosomiasis reference center in Angola, one hospital in South Sudan, and four hospitals in the Democratic Republic of the Congo between August 2005 and September 2009 to support the registration of Pafuramidine for treatment of first stage HAT in collaboration with the United States Food and Drug Administration. Patients were treated with either 100 mg of Pafuramidine orally twice a day for 10 days or 4 mg/kg pentamidine intramuscularly once daily for 7 days to assess the efficacy and safety of Pafuramidine versus pentamidine. Pregnant and lactating women as well as adolescents were included. The primary efficacy endpoint was the combined rate of clinical and parasitological cure at 12 months. The primary safety outcome was the frequency and severity of adverse events. The study was registered on the International Clinical Trials Registry Platform at www.clinicaltrials.gov with the number ISRCTN85534673. FINDINGS/CONCLUSIONS: The overall cure rate at 12 months was 89% in the Pafuramidine group and 95% in the pentamidine group; Pafuramidine was non-inferior to pentamidine as the upper bound of the 95% confidence interval did not exceed 15%. The safety profile of Pafuramidine was superior to pentamidine; however, 3 patients in the Pafuramidine group had glomerulonephritis or nephropathy approximately 8 weeks post-treatment. Two of these events were judged as possibly related to Pafuramidine. Despite good tolerability observed in preceding studies, the development program for Pafuramidine was discontinued due to delayed post-treatment toxicity.
Efficacy, Safety, and Dose of Pafuramidine, a New Oral Drug for Treatment of First Stage Sleeping Sickness, in a Phase 2a Clinical Study and Phase 2b Randomized Clinical Studies.[Pubmed:26881924]
PLoS Negl Trop Dis. 2016 Feb 16;10(2):e0004362.
BACKGROUND: Sleeping sickness (human African trypanosomiasis [HAT]) is caused by protozoan parasites and characterized by a chronic progressive course, which may last up to several years before death. We conducted two Phase 2 studies to determine the efficacy and safety of oral Pafuramidine in African patients with first stage HAT. METHODS: The Phase 2a study was an open-label, non-controlled, proof-of-concept study where 32 patients were treated with 100 mg of Pafuramidine orally twice a day (BID) for 5 days at two trypanosomiasis reference centers (Angola and the Democratic Republic of the Congo [DRC]) between August 2001 and November 2004. The Phase 2b study compared Pafuramidine in 41 patients versus standard pentamidine therapy in 40 patients. The Phase 2b study was open-label, parallel-group, controlled, randomized, and conducted at two sites in the DRC between April 2003 and February 2007. The Phase 2b study was then amended to add an open-label sequence (Phase 2b-2), where 30 patients received Pafuramidine for 10 days. The primary efficacy endpoint was parasitologic cure at 24 hours (Phase 2a) or 3 months (Phase 2b) after treatment completion. The primary safety outcome was the rate of occurrence of World Health Organization Toxicity Scale Grade 3 or higher adverse events. All subjects provided written informed consent. FINDINGS/CONCLUSION: Pafuramidine for the treatment of first stage HAT was comparable in efficacy to pentamidine after 10 days of dosing. The cure rates 3 months post-treatment were 79% in the 5-day Pafuramidine, 100% in the 7-day pentamidine, and 93% in the 10-day Pafuramidine groups. In Phase 2b, the percentage of patients with at least 1 treatment-emergent adverse event was notably higher after pentamidine treatment (93%) than Pafuramidine treatment for 5 days (25%) and 10 days (57%). These results support continuation of the development program for Pafuramidine into Phase 3.
Phase I/II evaluation of the prophylactic antimalarial activity of pafuramidine in healthy volunteers challenged with Plasmodium falciparum sporozoites.[Pubmed:19346370]
Am J Trop Med Hyg. 2009 Apr;80(4):528-35.
We evaluated the causal prophylactic antimalarial activity of a single oral dose of Pafuramidine (DB289), an experimental prodrug of active metabolite DB75, in a randomized, double-blind, placebo-controlled, outpatient study. Sixteen healthy volunteers were dosed and challenged in a single cohort. Subjects were randomly assigned to one of three treatment arms: 100 mg Pafuramidine eight days before challenge, 100 mg Pafuramidine the day before challenge, or placebo. Challenge was by the bites of Plasmodium falciparum-infected Anopheles gambiae. Malaria developed in 15 persons but did not develop in one person in the day -8 Pafuramidine treatment arm. Plasma levels of DB75 were lower than expected, and as intended were too low to provide suppressive prophylaxis at the earliest appearance of erythrocytic parasites. We conclude that a single dose of 100 mg Pafuramidine does not adequately protect non-immune individuals against P. falciparum and shows no clinically or statistically significant evidence of causal prophylactic activity.


