LuliconazoleAzole antifungal agent(interdigital tinea pedis) CAS# 187164-19-8 |
- HG-10-102-01
Catalog No.:BCC4271
CAS No.:1351758-81-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 187164-19-8 | SDF | Download SDF |
| PubChem ID | 3003141 | Appearance | Powder |
| Formula | C14H9Cl2N3S2 | M.Wt | 354.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NND 502 | ||
| Solubility | DMSO : ≥ 41 mg/mL (115.73 mM) *"≥" means soluble, but saturation unknown. | ||
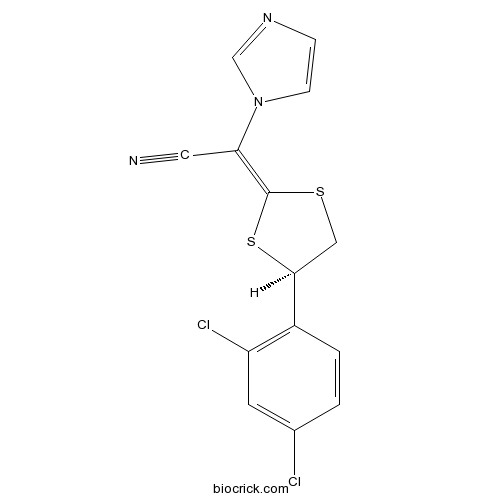
| Chemical Name | (2E)-2-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-ylacetonitrile | ||
| SMILES | C1C(SC(=C(C#N)N2C=CN=C2)S1)C3=C(C=C(C=C3)Cl)Cl | ||
| Standard InChIKey | YTAOBBFIOAEMLL-REQDGWNSSA-N | ||
| Standard InChI | InChI=1S/C14H9Cl2N3S2/c15-9-1-2-10(11(16)5-9)13-7-20-14(21-13)12(6-17)19-4-3-18-8-19/h1-5,8,13H,7H2/b14-12+/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Luliconazole(NND 502) is an azole antifungal indicated for the topical treatment of interdigital tinea pedis.
IC50 Value:
Target: Antifungal
Luliconazole is an antifungal that belongs to the azole class. Although the exact mechanism of action against dermatophytes is unknown, luliconazole appears to inhibit ergosterol synthesis by inhibiting the enzyme lanosterol demethylase. Inhibition of this enzyme’s activity by azoles results in decreased amounts of ergosterol, a constituent of fungal cell membranes, and a corresponding accumulation of lanosterol.
In a fertility study in rats, subcutaneous doses of 1, 5 and 25 mg/kg/day luliconazole were administered prior to and during mating and through early pregnancy. Treatment related effects on reproductive function were noted in females (decreased live embryos and decreased corpus luteum) at 5 and 25 mg/kg/day and males (decreased sperm counts) at 25 mg/kg/day. No treatment related effects on fertility or reproductive function were noted at 1 mg/kg/day (0.1X MRHD based on BSA comparisons). References: | |||||

Luliconazole Dilution Calculator

Luliconazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8226 mL | 14.1131 mL | 28.2263 mL | 56.4525 mL | 70.5657 mL |
| 5 mM | 0.5645 mL | 2.8226 mL | 5.6453 mL | 11.2905 mL | 14.1131 mL |
| 10 mM | 0.2823 mL | 1.4113 mL | 2.8226 mL | 5.6453 mL | 7.0566 mL |
| 50 mM | 0.0565 mL | 0.2823 mL | 0.5645 mL | 1.1291 mL | 1.4113 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2823 mL | 0.5645 mL | 0.7057 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Luliconazole(NND 502) is an azole antifungal indicated for the topical treatment of interdigital tinea pedis.
- Clauszoline M
Catalog No.:BCN4683
CAS No.:187110-72-1
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- N,N'-Bis(2-hydroxyethyl)oxamide
Catalog No.:BCC9061
CAS No.:1871-89-2
- Sinapine
Catalog No.:BCN1815
CAS No.:18696-26-9
- Pafuramidine
Catalog No.:BCC1832
CAS No.:186953-56-0
- 2B-(SP)
Catalog No.:BCC5817
CAS No.:186901-17-7
- Moxifloxacin HCl
Catalog No.:BCC2507
CAS No.:186826-86-8
- ML 10302 hydrochloride
Catalog No.:BCC7695
CAS No.:186826-17-5
- Ginsenoside Rg5
Catalog No.:BCN3551
CAS No.:186763-78-0
- Alisol A 24-acetate
Catalog No.:BCN2344
CAS No.:18674-16-3
- N6-methyladenosine (m6A)
Catalog No.:BCC6495
CAS No.:1867-73-8
- Ketamine hydrochloride
Catalog No.:BCC5982
CAS No.:1867-66-9
- Cyanidin-3-O-rutinoside chloride
Catalog No.:BCN3114
CAS No.:18719-76-1
- Oseltamivir acid
Catalog No.:BCC1826
CAS No.:187227-45-8
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Kimcuongin
Catalog No.:BCN7472
CAS No.:1872403-23-0
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- ER 50891
Catalog No.:BCC7783
CAS No.:187400-85-7
- Methylisopelletierine
Catalog No.:BCN1160
CAS No.:18747-42-7
- Sitoindoside I
Catalog No.:BCN1161
CAS No.:18749-71-8
- N-Aminophthalimide
Catalog No.:BCC9085
CAS No.:1875-48-5
- MaxiPost
Catalog No.:BCC7984
CAS No.:187523-35-9
- Ethyl rutinoside
Catalog No.:BCC8976
CAS No.:187539-57-7
Potent Activities of Novel Imidazoles Lanoconazole and Luliconazole against a Collection of Azole-Resistant and -Susceptible Aspergillus fumigatus Strains.[Pubmed:27572389]
Antimicrob Agents Chemother. 2016 Oct 21;60(11):6916-6919.
A collection of azole-susceptible (n = 141) and azole-resistant (n = 27) Aspergillus fumigatus isolates was tested against seven antifungal drugs, including the new imidazoles lanoconazole and Luliconazole. The Luliconazole and lanoconazole MIC90 values for the azole-susceptible strains were 0.001 mug/ml and 0.008 mug/ml, and those for the azole-resistant strains were 0.016 mug/ml and 0.032 mug/ml.
The efficacy of fractional carbon dioxide (CO2) laser combined with luliconazole 1% cream for the treatment of onychomycosis: A randomized, controlled trial.[Pubmed:27858846]
Medicine (Baltimore). 2016 Nov;95(44):e5141.
BACKGROUND: To evaluate the efficacy of fractional carbon dioxide (CO2) laser combined with Luliconazole 1% cream for the treatment of onychomycosis and to compare it with that of fractional CO2 laser alone. METHODS: This was a randomized, parallel group, 2-arm, positive-controlled, single-center, superiority trial with a 1:2 allocation ratio. Sixty patients with clinical and mycological diagnosis of onychomycosis were enrolled from the Dermatology Department of the First Affiliated Hospital of Nanjing Medical University in Nanjing, China from March 2015 to May 2015. Patients were randomized following simple randomization procedures (computerized random number generator) into 2 groups; L group only received 12 sessions of laser treatment at 2-week interval for 6 months, while L + D group received 12 sessions of laser treatment at 2-week interval combined with Luliconazole 1% cream once daily for 6 months. This was not a blind trial. The main outcome measures were the clinical efficacy rate (CER) assessed from the percentage of fully and >60% normal-appearing nails and the mycological clearance rate (MCR) assessed from the percentage of nails with negative fungal microscopy. There were no changes to trial outcome measures after the trial commenced. RESULTS: A total of 60 patients (N = 233 nails) completed treatments and follow-up, and were randomized and divided into 2 groups: L group (31 patients, N = 108 nails) and L + D group (29 patients, N = 115 nails). The CER and MCR of L + D group were 69.6% and 57.4%, respectively. L + D group showed significantly higher CER (69.6% vs 50.9%; chi = 8.1, P = 0.004) and MCR (57.4% vs 38.9%; chi = 7.6, P = 0.006) compared with those in L group. Some patients experienced mild pain during laser treatment, but there was no bleeding or oozing during or after treatment. There were no adverse effects reported during the observation period. CONCLUSION: Fractional CO2 laser treatment combined with 1% Luliconazole cream for 6 months was an effective and safe method for the treatment of onychomycosis, and had a higher efficacy than fractional CO2 laser treatment alone.
Efficacy and safety of luliconazole 5% nail solution for the treatment of onychomycosis: A multicenter, double-blind, randomized phase III study.[Pubmed:28332720]
J Dermatol. 2017 Jul;44(7):753-759.
Onychomycosis is a highly prevalent and intractable disease. The first-line treatment agents are oral preparations, but an effective topical medication has long been desired. The objective was to investigate the efficacy and safety of Luliconazole 5% nail solution, an imidazole antifungal agent, for the treatment of patients with onychomycosis. A multicenter, double-blind, randomized phase III study was conducted in Japanese patients with distal lateral subungual onychomycosis affecting the great toenails, with 20-50% clinical involvement. Patients were randomized (2:1) to Luliconazole or vehicle once daily for 48 weeks. The primary end-point was the complete cure rate (clinical cure [0% clinical involvement of the nail] plus mycological cure [negative results on direct microscopy]). The adverse event incidence was monitored to evaluate safety. The complete cure rate significantly favored Luliconazole (14.9%, 29/194 subjects) versus vehicle (5.1%, 5/99) (P = 0.012). Similarly, the negative direct microscopy rate was significantly higher with Luliconazole (45.4%, 79/174) than with vehicle (31.2%, 29/93) (P = 0.026). There were no serious adverse drug reactions. We conclude that once daily topical Luliconazole 5% nail solution demonstrated clinical efficacy and was confirmed to be well tolerated.


