Sitoindoside ICAS# 18749-71-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18749-71-8 | SDF | Download SDF |
| PubChem ID | 9832350 | Appearance | Powder |
| Formula | C51H90O7 | M.Wt | 815.3 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
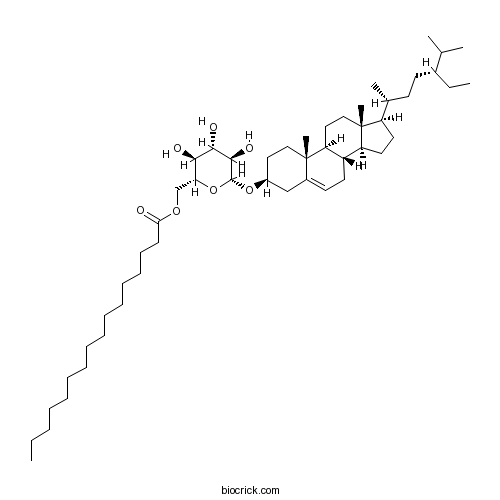
| Chemical Name | [(2R,3S,4S,5R,6R)-6-[[(3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-3,4,5-trihydroxyoxan-2-yl]methyl hexadecanoate | ||
| SMILES | CCCCCCCCCCCCCCCC(=O)OCC1C(C(C(C(O1)OC2CCC3(C4CCC5(C(C4CC=C3C2)CCC5C(C)CCC(CC)C(C)C)C)C)O)O)O | ||
| Standard InChIKey | JCLYMCVRBRHEHI-TWDDPRSNSA-N | ||
| Standard InChI | InChI=1S/C51H90O7/c1-8-10-11-12-13-14-15-16-17-18-19-20-21-22-45(52)56-34-44-46(53)47(54)48(55)49(58-44)57-39-29-31-50(6)38(33-39)25-26-40-42-28-27-41(51(42,7)32-30-43(40)50)36(5)23-24-37(9-2)35(3)4/h25,35-37,39-44,46-49,53-55H,8-24,26-34H2,1-7H3/t36-,37-,39+,40+,41-,42+,43+,44-,46-,47+,48-,49-,50+,51-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Sitoindoside I has anti-ulcerogenic activity. |
| Targets | Antifection |

Sitoindoside I Dilution Calculator

Sitoindoside I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2265 mL | 6.1327 mL | 12.2654 mL | 24.5308 mL | 30.6636 mL |
| 5 mM | 0.2453 mL | 1.2265 mL | 2.4531 mL | 4.9062 mL | 6.1327 mL |
| 10 mM | 0.1227 mL | 0.6133 mL | 1.2265 mL | 2.4531 mL | 3.0664 mL |
| 50 mM | 0.0245 mL | 0.1227 mL | 0.2453 mL | 0.4906 mL | 0.6133 mL |
| 100 mM | 0.0123 mL | 0.0613 mL | 0.1227 mL | 0.2453 mL | 0.3066 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methylisopelletierine
Catalog No.:BCN1160
CAS No.:18747-42-7
- ER 50891
Catalog No.:BCC7783
CAS No.:187400-85-7
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Kimcuongin
Catalog No.:BCN7472
CAS No.:1872403-23-0
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Oseltamivir acid
Catalog No.:BCC1826
CAS No.:187227-45-8
- Cyanidin-3-O-rutinoside chloride
Catalog No.:BCN3114
CAS No.:18719-76-1
- Luliconazole
Catalog No.:BCC1711
CAS No.:187164-19-8
- Clauszoline M
Catalog No.:BCN4683
CAS No.:187110-72-1
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- N,N'-Bis(2-hydroxyethyl)oxamide
Catalog No.:BCC9061
CAS No.:1871-89-2
- N-Aminophthalimide
Catalog No.:BCC9085
CAS No.:1875-48-5
- MaxiPost
Catalog No.:BCC7984
CAS No.:187523-35-9
- Ethyl rutinoside
Catalog No.:BCC8976
CAS No.:187539-57-7
- Fmoc-D-Arg(Pbf)-OH
Catalog No.:BCC3077
CAS No.:187618-60-6
- Tataramide B
Catalog No.:BCN3897
CAS No.:187655-56-7
- PNU 109291
Catalog No.:BCC7408
CAS No.:187665-60-7
- PNU 142633
Catalog No.:BCC7222
CAS No.:187665-65-2
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Serpentine
Catalog No.:BCN1162
CAS No.:18786-24-8
- Arachidonyl serotonin
Catalog No.:BCC7500
CAS No.:187947-37-1
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
Chemical and biological studies on Cichorium intybus L.[Pubmed:28629227]
Nat Prod Res. 2018 Jun;32(11):1343-1347.
Cichorium intybus L. (Asteraceae family) is a world-wide grown plant known as chicory. In traditional medicine, this plant is used as diuretic, anti-inflammatory, digestive, cardiotonic and liver tonic. Chromatographic purification of the supercritical fluid extract of aerial parts of C. intybus on silica gel column led to isolation of three compounds: new compound, 28beta-hydroxytaraxasterol (I), and two known compounds usnic acid (II) and beta-sitosterol (III). Purification of the ethanolic extract of aerial parts of this plant on silica gel column chromatography yielded four compounds: 1,3-dioleylglycerate (IV), Sitoindoside II (V), 11beta-13-dihydrolactucin (VI) and beta-sitosterol-3-O-glucoside (VII). The structures of the isolated compounds were determined by their 1D, 2D NMR and MS spectral data. All the fractions and isolated compounds were tested for cannabinoid and opioid receptor binding, as well as antibacterial, antifungal and antimalarial activities. Compound I showed moderate activity (60.5% displacement) towards CB1 receptor.
Delavayol, a novel sesquiterpene from Incarvillea delavayi Bureau et Franchet.[Pubmed:20496228]
Nat Prod Res. 2010 Jun;24(10):915-9.
To investigate the chemical constituents from Incarvillea delavayi Bureau et Franchet, a new sesquiterpene, named delavayol, together with three known ones, was isolated by column chromatography. Spectroscopic and chemical evidence revealed the structures to be 8beta,9beta-dihydroxy-1(10)-eremophiliene-11,12-diol (1), oleanolic acid (2), myrianthic acid (3), and Sitoindoside I (4). Compounds 3 and 4 were isolated from the genus Incarvillea for the first time.
Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves.[Pubmed:14575818]
Life Sci. 2003 Nov 21;74(1):125-32.
Ayurvedic medicines prepared in India consist of Withania somnifera roots as one of the main ingredients. It is consumed as a dietary supplement around the world. The leaves of W. somnifera were used in the treatment of tumors and inflammation in several Asian countries. We have isolated twelve withanolides such as withaferin A (1), Sitoindoside IX (2), 4-(1-hydroxy-2, 2-dimethylcyclpropanone)-2, 3-dihydrowithaferin A (3), 2, 3-dihydrowithaferin A (4), 24, 25-dihydro-27-desoxywithaferin A (5), physagulin D (1-->6)-beta-D-glucopyranosyl- (1-->4)-beta-D-glucopyranoside (6), 27-O-beta-D-glucopyranosylphysagulin D (7), physagulin D (8), withanoside IV (9), and 27-O-beta-D-glucopyranosylviscosalactone B (10), 4, 16-dihydroxy-5beta, 6beta-epoxyphysagulin D (11), viscosalactone B (12) from the leaves of this species. Compounds 1-12 and diacetylwithaferin A (13) were tested for their antiproliferative activity on NCI-H460 (Lung), HCT-116 (Colon), SF-268 (Central Nervous System; CNS and MCF-7 (Breast) human tumor cell lines. The inhibitory concentration to afford 50% cell viability (IC50) for these compounds was determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. Withaferin A and its derivatives exhibited inhibitory concentrations (50%) ranging from 0.24 +/- 0.01 to 11.6 +/- 1.9 microg/mL. Viscosalactone B (12) showed the 50% inhibition at concentrations ranging from 0.32 +/- 0.05 to 0.47 +/- 0.15 microg/mL whereas its 27-O-glucoside derivative (10) exhibited IC50 between 7.9 +/- 2.9 and 17.3 +/- 3.9 microg/ml. However, Physagulin D type withanolides showed either weak or no activity at 30 microg/mL. Therefore, incorporation of withanolides in the diet may prevent or decrease the growth of tumors in human.
Discovery of natural product chemopreventive agents utilizing HL-60 cell differentiation as a model.[Pubmed:7762989]
Anticancer Res. 1995 Mar-Apr;15(2):233-9.
Terminal differentiation of human promyelocytic leukemia (HL-60) cells can be induced by a variety of chemical agents and this process can be monitored readily by the generation of morphologically, histochemically, and functionally mature granulocytes and monocytes/macrophages. The availability of this model has heightened interest in the possible therapeutic role of inducers of myeloid differentiation for the treatment of leukemia and other neoplasms. In addition, however, potent cancer chemopreventive agents induce HL-60 cell differentiation at very low dose levels. Thus, as part of our search for natural product chemopreventive agents, extracts derived from nearly 400 plants were tested for their potential to induce HL-60 cell differentiation. As a result, 17 plant extracts were judged to be active (ED50 values < or = 4 micrograms/ml). One of most potent leads was an extract derived from Dirca occidentalis Gray (Thymelaeaceae) (ED50, 0.14 micrograms/ml), and bioassay-guided fractionation led to the identification of genkwanin (I), (+/-)-lariciresinol (II) and Sitoindoside II (IV) as active principles, with ED50 values of 18.3, 1.1 and 0.069 microM, respectively. Based on these data, we conclude that the HL-60 cell differentiation system is a valid and useful model for the discovery of natural product cancer chemopreventive or chemotherapeutic agents.


