SerpentineCAS# 18786-24-8 |
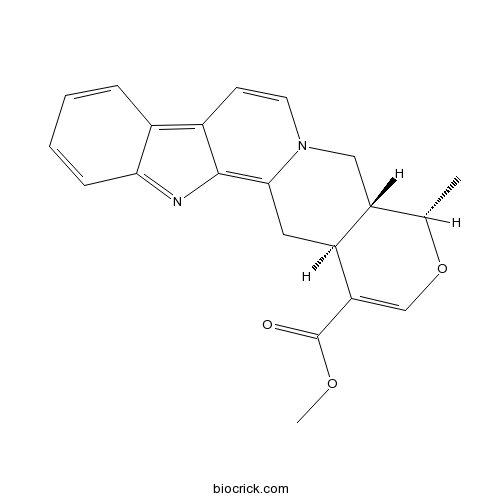
- Alstonine
Catalog No.:BCN4606
CAS No.:642-18-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18786-24-8 | SDF | Download SDF |
| PubChem ID | 73073 | Appearance | Yellow powder |
| Formula | C21H21N2O3 | M.Wt | 349.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (15S,20R)-16-methyl-17-oxa-3-aza-13-azoniapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-1(13),2(10),4,6,8,11,18-heptaene-19-carboxylate | ||
| SMILES | CC1C2C[N+]3=C(CC2C(=CO1)C(=O)OC)C4=C(C=C3)C5=CC=CC=C5N4 | ||
| Standard InChIKey | WYTGDNHDOZPMIW-AGIABQAESA-O | ||
| Standard InChI | InChI=1S/C21H20N2O3/c1-12-16-10-23-8-7-14-13-5-3-4-6-18(13)22-20(14)19(23)9-15(16)17(11-26-12)21(24)25-2/h3-8,11-12,15-16H,9-10H2,1-2H3/p+1/t12?,15-,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Analysis of serpentine polymorphs in investigations of natural occurrences of asbestos.[Pubmed: 25942071]Environ Sci Process Impacts. 2015 May 5.This work investigates potential analytical variability in environmental investigations of natural occurrences of asbestos (NOA) due to intergrown Serpentine minerals.
A facile route to preparation of high purity nanoporous silica from acid-leached residue of serpentine.[Pubmed: 25924349]J Nanosci Nanotechnol. 2014 Sep;14(9):6915-22.As the current cost of mineral carbonation is too high for an economically viable industrial process, it is desirable to produce value-added products from CO2 mineralization process.
Methodologies for determining the sources, characteristics, distribution, and abundance of asbestiform and nonasbestiform amphibole and serpentine in ambient air and water.[Pubmed: 25825806]J Toxicol Environ Health B Crit Rev. 2015;18(1):1-42.
|

Serpentine Dilution Calculator

Serpentine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.862 mL | 14.3102 mL | 28.6205 mL | 57.241 mL | 71.5512 mL |
| 5 mM | 0.5724 mL | 2.862 mL | 5.7241 mL | 11.4482 mL | 14.3102 mL |
| 10 mM | 0.2862 mL | 1.431 mL | 2.862 mL | 5.7241 mL | 7.1551 mL |
| 50 mM | 0.0572 mL | 0.2862 mL | 0.5724 mL | 1.1448 mL | 1.431 mL |
| 100 mM | 0.0286 mL | 0.1431 mL | 0.2862 mL | 0.5724 mL | 0.7155 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
- PNU 142633
Catalog No.:BCC7222
CAS No.:187665-65-2
- PNU 109291
Catalog No.:BCC7408
CAS No.:187665-60-7
- Tataramide B
Catalog No.:BCN3897
CAS No.:187655-56-7
- Fmoc-D-Arg(Pbf)-OH
Catalog No.:BCC3077
CAS No.:187618-60-6
- Ethyl rutinoside
Catalog No.:BCC8976
CAS No.:187539-57-7
- MaxiPost
Catalog No.:BCC7984
CAS No.:187523-35-9
- N-Aminophthalimide
Catalog No.:BCC9085
CAS No.:1875-48-5
- Sitoindoside I
Catalog No.:BCN1161
CAS No.:18749-71-8
- Methylisopelletierine
Catalog No.:BCN1160
CAS No.:18747-42-7
- ER 50891
Catalog No.:BCC7783
CAS No.:187400-85-7
- Arachidonyl serotonin
Catalog No.:BCC7500
CAS No.:187947-37-1
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
- Acetylcorynoline
Catalog No.:BCN1239
CAS No.:18797-80-3
- WKYMVM trifluoroacetate salt
Catalog No.:BCC5815
CAS No.:187986-11-4
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- Bikinin
Catalog No.:BCC5582
CAS No.:188011-69-0
- Abacavir sulfate
Catalog No.:BCC5023
CAS No.:188062-50-2
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
- NocII
Catalog No.:BCC5704
CAS No.:188119-47-3
Methodologies for determining the sources, characteristics, distribution, and abundance of asbestiform and nonasbestiform amphibole and serpentine in ambient air and water.[Pubmed:25825806]
J Toxicol Environ Health B Crit Rev. 2015;18(1):1-42.
Anthropogenic and nonanthropogenic (erosion) processes contribute to the continuing presence of asbestos and nonasbestos elongated mineral particles (EMP) of amphibole and Serpentine in air and water of urban, rural, and remote environments. The anthropogenic processes include disturbance and deterioration of asbestos-containing materials, mining of amphibole- and Serpentine-bearing rock, and disturbance of soils containing amphibole and Serpentine. Atmospheric dispersal processes can transport EMP on a global scale. There are many methods of establishing the abundance of EMP in air and water. EMP include cleavage fragments, fibers, asbestos, and other asbestiform minerals, and the methods employed do not critically distinguish among them. The results of most of the protocols are expressed in the common unit of fibers per square centimeter; however, seven different definitions for the term "fiber" are employed and the results are not comparable. The phase-contrast optical method used for occupational monitoring cannot identify particles being measured, and none of the methods distinguish amphibole asbestos from other EMP of amphibole. Measured ambient concentrations of airborne EMP are low, and variance may be high, even for similar environments, yielding data of questionable value for risk assessment. Calculations based on the abundance of amphibole-bearing rock and estimates of asbestos in the conterminous United States suggest that amphibole may be found in 6-10% of the land area; nonanthropogenic erosional processes might produce on the order of 400,000 tons or more of amphibole per year, and approximately 50 g asbestos/km(2)/yr; and the order of magnitude of the likelihood of encountering rock bearing any type of asbestos is approximately 0.0001.
Analysis of serpentine polymorphs in investigations of natural occurrences of asbestos.[Pubmed:25942071]
Environ Sci Process Impacts. 2015 May;17(5):985-96.
This work investigates potential analytical variability in environmental investigations of natural occurrences of asbestos (NOA) due to intergrown Serpentine minerals. Franciscan complex and serpentinite rock samples were obtained from likely NOA sites in coastal Northern California with geographic information system (GIS) maps, then analyzed using polarized light microscopy (PLM), transmission electron microscopy with energy-dispersive X-ray analysis and selected area electron diffraction (TEM/SAED/EDS), and environmental scanning electron microscopy with EDS (ESEM/EDS). Non-asbestos Serpentine fibers were superficially similar to chrysotile but were differentiated quickly using TEM morphology criteria and reference SAED overlays. 94 NOA fibers were classified as asbestiform chrysotile (62%), polygonal Serpentine (34%), lizardite scrolls (2%), and lizardite laths (2%). Chrysotile fibril widths (mean = 42 nm) were significantly different from those of polygonal Serpentine and lizardite laths (167 and 505 nm, respectively), but not lizardite scrolls (37 nm). Due to differing preparations and microscope resolutions, TEM analyses investigated a distinct, smaller population of particles (0.01-10 mum) than did PLM analyses (10-100 mum). A higher proportion of asbestiform phases in the finer fraction could potentially bias TEM bulk percent asbestos determinations. ESEM/EDS of intermediate particle size ranges revealed 20-200 mum, elongated particles with intermixed asbestiform and non-asbestiform structures on their surfaces. These particles were too thick and complex to be resolved by PLM, and too massive to be detected by TEM. These large particles are likely to exist in samples prepared by mechanical crushing or grinding, but are not likely to be generated by "releasable asbestos" methods.
A facile route to preparation of high purity nanoporous silica from acid-leached residue of serpentine.[Pubmed:25924349]
J Nanosci Nanotechnol. 2014 Sep;14(9):6915-22.
As the current cost of mineral carbonation is too high for an economically viable industrial process, it is desirable to produce value-added products from CO2 mineralization process. In this work, a facile and cost-effective process was developed for the production of high purity SiO2 from acid-leached Serpentine residue. The Si extraction rate is fast even under ambient conditions due to the highly defective structure of the residue. The reaction kinetics were studied and it was found that the Si extraction rate was under a combination of chemical reaction control and film diffusion control. The SiO2 sample prepared has high purity with a nanoporous structure, which renders it a potential candidate for applications such as an adsorbent and a catalyst support.


