BikininGSK3 inhibitor and Brassinosteroid activator CAS# 188011-69-0 |
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 188011-69-0 | SDF | Download SDF |
| PubChem ID | 647833 | Appearance | Powder |
| Formula | C9H9BrN2O3 | M.Wt | 273.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Abrasin | ||
| Solubility | DMSO : ≥ 42 mg/mL (153.80 mM) *"≥" means soluble, but saturation unknown. | ||
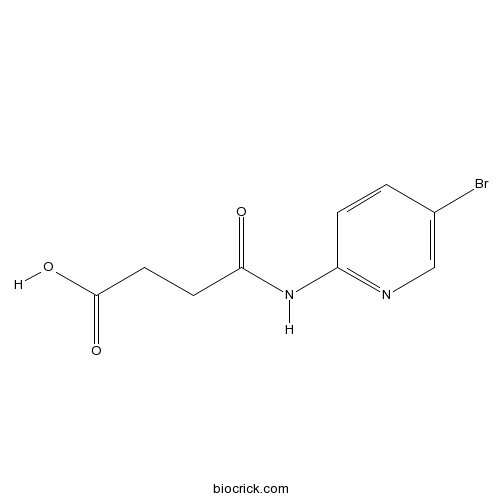
| Chemical Name | 4-[(5-bromopyridin-2-yl)amino]-4-oxobutanoic acid | ||
| SMILES | C1=CC(=NC=C1Br)NC(=O)CCC(=O)O | ||
| Standard InChIKey | XFYYQDHEDOXWGA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H9BrN2O3/c10-6-1-2-7(11-5-6)12-8(13)3-4-9(14)15/h1-2,5H,3-4H2,(H,14,15)(H,11,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bikinin Dilution Calculator

Bikinin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6619 mL | 18.3097 mL | 36.6193 mL | 73.2386 mL | 91.5483 mL |
| 5 mM | 0.7324 mL | 3.6619 mL | 7.3239 mL | 14.6477 mL | 18.3097 mL |
| 10 mM | 0.3662 mL | 1.831 mL | 3.6619 mL | 7.3239 mL | 9.1548 mL |
| 50 mM | 0.0732 mL | 0.3662 mL | 0.7324 mL | 1.4648 mL | 1.831 mL |
| 100 mM | 0.0366 mL | 0.1831 mL | 0.3662 mL | 0.7324 mL | 0.9155 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bikini is a strong activator of Brassinosteroid (BR) signaling [1] and an inhibitor of glycogen synthase kinase 3 proteins (GSK3) [2].
BRs are a group of polyhydroxylated steroid hormones implicated in multiple developmental processes, including stem elongation, leaf expansion, vascular development, seed germination, and resistance to biotic and abiotic stresses. Genetic defects in biosynthesis or perception of BRs resulted in dwarfism, dark-green and curled leaves, reduced seed germination and fertility, and de-etiolation in the dark. [1]
Bikinin is the first nonsteroidal molecule to modulate the BR signaling cascade downstream of the BRI1 receptor. Bikinin directly targeted BIN2 and activated BR-dependent gene expression. Application of bikini partially restored the phenotype of the bri1 loss-of-function mutant and the bin2 gain-of-function mutant to wild-type. Bikinin clearly rescued the dwarfism of the hypocotyls and the petioles of the bin2 gain-of-function mutant. Bikinin are fully rescuing the BR-related phenotypes of this mutation while having no effect on the auxin-related phenotypes. [1]
Bikinin treatment also induced tracheary element differentiation in Nicotiana benthamiana leaf disks, this leaf-disk culture system may be applicable to different plant species. [2]
References:
[1]. De Rybel B, Audenaert D, Vert G et al. Chemical inhibition of a subset of Arabidopsis thaliana GSK3-like kinases activates brassinosteroid signaling. Chem Biol. 2009 Jun 26;16(6):594-604.
[2]. Kondo Y, Fujita T, Sugiyama M, Fukuda H. A Novel System for Xylem Cell Differentiation in Arabidopsis thaliana. Mol Plant. 2014 Dec 13. pii: S1674-2052(14)00026-4.
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- WKYMVM trifluoroacetate salt
Catalog No.:BCC5815
CAS No.:187986-11-4
- Acetylcorynoline
Catalog No.:BCN1239
CAS No.:18797-80-3
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
- Arachidonyl serotonin
Catalog No.:BCC7500
CAS No.:187947-37-1
- Serpentine
Catalog No.:BCN1162
CAS No.:18786-24-8
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
- PNU 142633
Catalog No.:BCC7222
CAS No.:187665-65-2
- PNU 109291
Catalog No.:BCC7408
CAS No.:187665-60-7
- Abacavir sulfate
Catalog No.:BCC5023
CAS No.:188062-50-2
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
- NocII
Catalog No.:BCC5704
CAS No.:188119-47-3
- H-Ser(tBu)-OH
Catalog No.:BCC3032
CAS No.:18822-58-7
- H-Tyr(tBu)-OH
Catalog No.:BCC3129
CAS No.:18822-59-8
- (±)-Octanoylcarnitine chloride
Catalog No.:BCC6715
CAS No.:18822-86-1
- Methylproamine
Catalog No.:BCC1741
CAS No.:188247-01-0
- (±)-Propionylcarnitine chloride
Catalog No.:BCC6719
CAS No.:18828-58-5
- 8alpha-(2-Methylacryloyloxy)hirsutinolide
Catalog No.:BCN7109
CAS No.:188293-70-1
- Californidine
Catalog No.:BCC8137
CAS No.:18830-99-4
- Massonianoside B
Catalog No.:BCN1164
CAS No.:188300-19-8
Bikinin-like inhibitors targeting GSK3/Shaggy-like kinases: characterisation of novel compounds and elucidation of their catabolism in planta.[Pubmed:24947596]
BMC Plant Biol. 2014 Jun 19;14:172.
BACKGROUND: Plant GSK-3/Shaggy-like kinases are key players in brassinosteroid (BR) signalling which impact on plant development and participate in response to wounding, pathogens and salt stress. Bikinin was previously identified in a chemical genetics screen as an inhibitor targeting these kinases. To dissect the structural elements crucial for inhibition of GSK-3/Shaggy-like kinases by Bikinin and to isolate more potent compounds we synthesised a number of related substances and tested their inhibitory activity in vitro and in vivo using Arabidopsis thaliana. RESULTS: A pyridine ring with an amido succinic acid residue in position 2 and a halogen in position 5 were crucial for inhibitory activity. The compound with an iodine substituent in position 5, denoted iodoBikinin, was most active in inhibiting BIN2 activity in vitro and efficiently induced brassinosteroid-like responses in vivo. Its methyl ester, methyliodoBikinin, showed improved cell permeability, making it highly potent in vivo although it had lower activity in vitro. HPLC analysis revealed that the methyl residue was rapidly cleaved off in planta liberating active iodoBikinin. In addition, we provide evidence that iodoBikinin and Bikinin are inactivated in planta by conjugation with glutamic acid or malic acid and that the latter process is catalysed by the malate transferase SNG1. CONCLUSION: Brassinosteroids participate in regulation of many aspects of plant development and in responses to environmental cues. Thus compounds modulating their action are valuable tools to study such processes and may be an interesting opportunity to modify plant growth and performance in horticulture and agronomy. Here we report the development of Bikinin derivatives with increased potency that can activate BR signalling and mimic BR action. MethyliodoBikinin was 3.4 times more active in vivo than Bikinin. The main reason for the superior activity of methyliodoBikinin, the most potent compound, is its enhanced plant tissue permeability. Inactivation of Bikinin and its derivatives in planta involves SNG1, which constitutes a novel pathway for modification of xenobiotic compounds.


