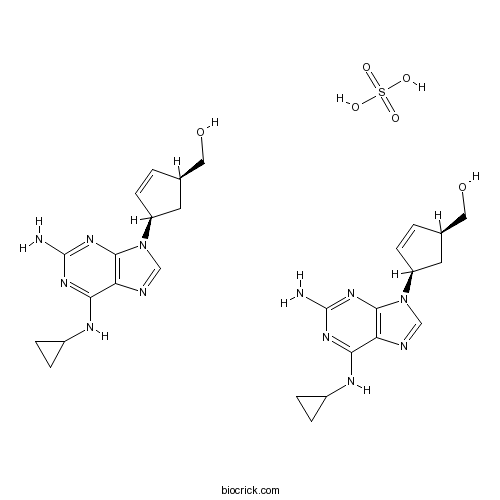
Abacavir sulfateReverse transcriptase inhibitor; antiretroviral CAS# 188062-50-2 |
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Tolcapone
Catalog No.:BCC2334
CAS No.:134308-13-7
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Lonafarnib
Catalog No.:BCC2331
CAS No.:193275-84-2
- FK866 (APO866)
Catalog No.:BCC2332
CAS No.:658084-64-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 188062-50-2 | SDF | Download SDF |
| PubChem ID | 441384 | Appearance | Powder |
| Formula | C14H20N6O5S | M.Wt | 384.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (74.54 mM; Need ultrasonic) | ||
| Chemical Name | [(1S,4R)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol;sulfuric acid | ||
| SMILES | C1CC1NC2=NC(=NC3=C2N=CN3C4CC(C=C4)CO)N.C1CC1NC2=NC(=NC3=C2N=CN3C4CC(C=C4)CO)N.OS(=O)(=O)O | ||
| Standard InChIKey | WMHSRBZIJNQHKT-FFKFEZPRSA-N | ||
| Standard InChI | InChI=1S/2C14H18N6O.H2O4S/c2*15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21;1-5(2,3)4/h2*1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19);(H2,1,2,3,4)/t2*8-,10+;/m11./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive reverse transcriptase inhibitor; inhibits HIV replication. Orally active and brain penetrant. Antiretroviral agent. |

Abacavir sulfate Dilution Calculator

Abacavir sulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6014 mL | 13.0069 mL | 26.0139 mL | 52.0278 mL | 65.0347 mL |
| 5 mM | 0.5203 mL | 2.6014 mL | 5.2028 mL | 10.4056 mL | 13.0069 mL |
| 10 mM | 0.2601 mL | 1.3007 mL | 2.6014 mL | 5.2028 mL | 6.5035 mL |
| 50 mM | 0.052 mL | 0.2601 mL | 0.5203 mL | 1.0406 mL | 1.3007 mL |
| 100 mM | 0.026 mL | 0.1301 mL | 0.2601 mL | 0.5203 mL | 0.6503 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Abacavir sulfate (ABC) is a powerful nucleoside analog reverse transcriptase inhibitor (NRTI) used to treat HIV and AIDS.
- Bikinin
Catalog No.:BCC5582
CAS No.:188011-69-0
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- WKYMVM trifluoroacetate salt
Catalog No.:BCC5815
CAS No.:187986-11-4
- Acetylcorynoline
Catalog No.:BCN1239
CAS No.:18797-80-3
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
- Arachidonyl serotonin
Catalog No.:BCC7500
CAS No.:187947-37-1
- Serpentine
Catalog No.:BCN1162
CAS No.:18786-24-8
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
- PNU 142633
Catalog No.:BCC7222
CAS No.:187665-65-2
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
- NocII
Catalog No.:BCC5704
CAS No.:188119-47-3
- H-Ser(tBu)-OH
Catalog No.:BCC3032
CAS No.:18822-58-7
- H-Tyr(tBu)-OH
Catalog No.:BCC3129
CAS No.:18822-59-8
- (±)-Octanoylcarnitine chloride
Catalog No.:BCC6715
CAS No.:18822-86-1
- Methylproamine
Catalog No.:BCC1741
CAS No.:188247-01-0
- (±)-Propionylcarnitine chloride
Catalog No.:BCC6719
CAS No.:18828-58-5
- 8alpha-(2-Methylacryloyloxy)hirsutinolide
Catalog No.:BCN7109
CAS No.:188293-70-1
- Californidine
Catalog No.:BCC8137
CAS No.:18830-99-4
- Massonianoside B
Catalog No.:BCN1164
CAS No.:188300-19-8
- Isodomoic acid G
Catalog No.:BCN1839
CAS No.:188346-81-8
Safety analysis of Ziagen(R) (abacavir sulfate) in postmarketing surveillance in Japan.[Pubmed:24585486]
Pharmacoepidemiol Drug Saf. 2014 Apr;23(4):361-71.
PURPOSE: Abacavir is a nucleoside reverse transcriptase inhibitor indicated for human immunodeficiency virus (HIV) infection. In Japan, Ziagen(R) (300-mg Abacavir sulfate) has been marketed since 1999. To obtain safety data on Ziagen, a mandatory postmarketing surveillance was conducted between September 1999 and September 2009. METHODS: A joint survey [HIV-related Drug Surveys (HRD)] has been conducted involving manufacturers of drugs for HIV treatment in Japan. Safety data from total 643 cases (1345.7 person-years) registered to the HRD surveys and received Ziagen were obtained. Adverse drug reaction (ADR) was defined as adverse event of which association with abacavir could not be "ruled out." RESULTS: It was found that the overall frequency of ADR was 47.6% (306/643); the common ADRs were "hyperlipidemia," "nausea," "increased gamma-glutamyltransferase level," "increased blood triglycerides," "abnormal hepatic function," and so on. Serious adverse events were reported in 65 subjects; however, none of the three fatal cases were clearly associated with Ziagen use. The survey-defined hypersensitivity has been infrequently reported in 15 subjects (2.3%). Although some studies had indicated of the association between abacavir and myocardial infarction, no ischemic heart diseases were reported in the present survey. Two of the three pregnant cases delivered normal neonates (one induced abortion). CONCLUSIONS: During the mandatory postmarketing survey of Ziagen, there were no cases of ischemic heart diseases, and the incidence of hypersensitivity was considerably low. These indicated that abacavir can be safely used in Japanese HIV+ population. However, the safety profile of Ziagen should be continued to be monitored through pharmacovigilance.
Stability behaviour of antiretroviral drugs and their combinations. 5: Characterization of novel degradation products of abacavir sulfate by mass and nuclear magnetic resonance spectrometry.[Pubmed:27889293]
J Pharm Biomed Anal. 2017 Feb 5;134:372-384.
In the present study, degradation behaviour of Abacavir sulfate was evaluated in solution and solid stress conditions. Solution state studies resulted in formation of eleven degradation products; of which two were also formed on solid stress. The same were separated by high performance liquid chromatography. They were characterized using liquid chromatography-high resolution mass spectrometry, liquid chromatography-multistage mass spectrometry and hydrogen/deuterium exchange mass spectrometry data. Additionally, seven degradation products were isolated and subjected to 1D and 2D nuclear magnetic resonance studies for their structural confirmation.
Evaluation of the Lipid Concentrations after Switching from Antiretroviral Drug Tenofovir Disoproxil Fumarate/Emtricitabine to Abacavir Sulfate/Lamivudine in Virologically-suppressed Human Immunodeficiency Virus-infected Patients.[Pubmed:27904105]
Intern Med. 2016;55(23):3435-3440.
Objective Recently, tenofovir disoproxil fumatate (TDF)-related side effects, such as renal nephrotoxicity and reduction of bone mineral density, have been reported. Consequently, increased switching from fixed-dose tablet TDF and emtricitabine (TDF/FTC) to abacavir and lamivudine (ABC/3TC) has occurred. Interestingly, while TDF has a lipid-lowering property, one of the ABC-related side effects is hyperlipidemia. Therefore, such switching could cause lipid elevation. To evaluate the change in lipid levels associated with switching from TDF/FTC to ABC/3TC in virologically-suppressed human immunodeficiency virus (HIV)-infected patients. Methods This is a retrospective, single-center study. We included the HIV-infected patients whose therapy included a drug switch from TDF/FTC to ABC/3TC between September 2009 and December 2012 at Ryukyu University Hospital. The exclusion criteria were HIV-RNA >40 copies/mL on the switching day, and a documented therapy change to a lipid-lowering agent or any other antiretroviral agents within 3 months before or after switching. We compared the low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), and triglyceride (TG) levels before switching to three months after. Results A total of 18 patients met the inclusion criteria. The LDL, HDL, and TC levels significantly increased three months following the switch (p<0.05), with median (interquartile range) values of 17 (7, 32), 6 (2, 13), and 27 (10, 45) mg/dL, respectively. The TG values did not markedly change. Conclusion Switching from TDF/FTC to ABC/3TC resulted in significantly increased LDL, HDL, and TC levels.
Safety analysis of Epzicom(R) (lamivudine/abacavir sulfate) in post-marketing surveillance in Japan.[Pubmed:24590575]
Pharmacoepidemiol Drug Saf. 2014 Apr;23(4):372-81.
PURPOSE: To obtain safety and effectiveness data on a combined anti-HIV drug, Epzicom (abacavir 600 mg/lamivudine 300 mg), a post-marketing surveillance on Epzicom that was required by the Japanese regulatory authority was conducted between January 2005 and December 2010. METHODS: A joint survey (HIV-related drug [HRD] survey) has been conducted involving manufacturers of drugs for treatment of HIV infection in Japan. Safety and effectiveness data from total 624 cases (1107.3 person-years) registered to the HRD surveys and received Epzicom were obtained. Adverse drug reactions (ADRs) were defined as adverse events (AE) of which association with Epzicom could not be 'ruled out'. RESULTS: It was found that the incidence of ADR was 32.4% (202/624 cases) on the case basis. In addition, the frequently reported ADR included hyperlipidaemia (59 cases), hypertriglyceridaemia (21 cases), blood bilirubin increased (19 cases), gamma-glutamyltransferase increase (14 cases), blood triglyceride increase (14 cases) and rash (14 cases). Serious AEs were seen in 19 patients (30 events), including one death (no evident association with Epzicom). There were four cases (0.6%) of survey-defined 'hypersensitivity', and the incidence was 0.9% (4/445) among abacavir naive patients; none of which was reported as serious. No case of myocardial infarction was reported. One pregnant case who delivered a normal baby by caesarean section was reported to have experienced aggravation of anaemia and nausea. CONCLUSIONS: The post-marketing surveillance indicated that the incidence of both ischaemic heart disease and hypersensitivity associated with Epzicom was considerably low, suggesting that this drug can be safely used in the Japanese population.
N-terminal valine adduct from the anti-HIV drug abacavir in rat haemoglobin as evidence for abacavir metabolism to a reactive aldehyde in vivo.[Pubmed:22725138]
Br J Pharmacol. 2012 Nov;167(6):1353-61.
BACKGROUND AND PURPOSE: The aim of this study was to obtain evidence for the activation of the nucleoside reverse transcriptase inhibitor abacavir to reactive aldehyde metabolites in vivo. Protein haptenation by these reactive metabolites may be a factor in abacavir-induced toxic events. EXPERIMENTAL APPROACH: The formation of N-terminal valine adducts from the abacavir-derived aldehydes was investigated in the haemoglobin of Wistar rats treated with eight daily doses (120 mg.kg(-1)) of abacavir. The analyses were conducted by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry upon comparison with synthetic standards. KEY RESULTS: An N-terminal valine haemoglobin adduct derived from an alpha,beta-unsaturated aldehyde metabolite of abacavir was identified in vivo for the first time. CONCLUSIONS AND IMPLICATIONS: This preliminary work on abacavir metabolism provides the first unequivocal evidence for the formation of an alpha,beta-unsaturated aldehyde metabolite in vivo and of its ability to form haptens with proteins. The methodology described herein can be used to assess the formation of this metabolite in human samples and has the potential to become a valuable pharmacological tool for mechanistic studies of abacavir toxicity. In fact, the simplicity of the method suggests that the abacavir adduct with the N-terminal valine of haemoglobin could be used to investigate abacavir-induced toxicity for accurate risk/benefit estimations.
Resistance profile of the human immunodeficiency virus type 1 reverse transcriptase inhibitor abacavir (1592U89) after monotherapy and combination therapy. CNA2001 Investigative Group.[Pubmed:10720512]
J Infect Dis. 2000 Mar;181(3):912-20.
Abacavir (1592U89) is a nucleoside inhibitor of human immunodeficiency virus (HIV) type 1 reverse transcriptase (RT). Resistance to abacavir was studied with abacavir alone and with abacavir in combination with other nucleoside analogues in cell culture, in virus isolates from zidovudine/lamivudine clinical trials, and in the first dose-escalating 12-week clinical trial (CNA2001) to evaluate abacavir clinical potency. Abacavir alone in vitro selected for mutations at HIV RT codons K65R, L74V, Y115F, and M184V. However, abacavir combined with zidovudine selected against virus with the M184V mutation. Abacavir therapy in vivo resulted in large decreases in HIV load (>1 log), even in 1 subject who had the M184V mutation at baseline. A total of 51% of subjects showed new mutations at any of codons K65R, L74V, and M184V after abacavir monotherapy, compared with 11% who received zidovudine/abacavir. Small changes (2- to 4-fold) in abacavir susceptibility were detected. On stopping therapy, reselection of the pretherapy sequence occurred within 4 weeks.
1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity.[Pubmed:9145874]
Antimicrob Agents Chemother. 1997 May;41(5):1082-93.
1592U89, (-)-(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclo pentene-1-methanol, is a carbocyclic nucleoside with a unique biological profile giving potent, selective anti-human immunodeficiency virus (HIV) activity. 1592U89 was selected after evaluation of a wide variety of analogs containing a cyclopentene substitution for the 2'-deoxyriboside of natural deoxynucleosides, optimizing in vitro anti-HIV potency, oral bioavailability, and central nervous system (CNS) penetration. 1592U89 was equivalent in potency to 3'-azido-3'-deoxythymidine (AZT) in human peripheral blood lymphocyte (PBL) cultures against clinical isolates of HIV type 1 (HIV-1) from antiretroviral drug-naive patients (average 50% inhibitory concentration [IC50], 0.26 microM for 1592U89 and 0.23 microM for AZT). 1592U89 showed minimal cross-resistance (approximately twofold) with AZT and other approved HIV reverse transcriptase (RT) inhibitors. 1592U89 was synergistic in combination with AZT, the nonnucleoside RT inhibitor nevirapine, and the protease inhibitor 141W94 in MT4 cells against HIV-1 (IIIB). 1592U89 was anabolized intracellularly to its 5'-monophosphate in CD4+ CEM cells and in PBLs, but the di- and triphosphates of 1592U89 were not detected. The only triphosphate found in cells incubated with 1592U89 was that of the guanine analog (-)-carbovir (CBV). However, the in vivo pharmacokinetic, distribution, and toxicological profiles of 1592U89 were distinct from and improved over those of CBV, probably because CBV itself was not appreciably formed from 1592U89 in cells or animals (<2%). The 5'-triphosphate of CBV was a potent, selective inhibitor of HIV-1 RT, with Ki values for DNA polymerases (alpha, beta, gamma, and epsilon which were 90-, 2,900-, 1,200-, and 1,900-fold greater, respectively, than for RT (Ki, 21 nM). 1592U89 was relatively nontoxic to human bone marrow progenitors erythroid burst-forming unit and granulocyte-macrophage CFU (IC50s, 110 microM) and human leukemic and liver tumor cell lines. 1592U89 had excellent oral bioavailability (105% in the rat) and penetrated the CNS (rat brain and monkey cerebrospinal fluid) as well as AZT. Having demonstrated an excellent preclinical profile, 1592U89 has progressed to clinical evaluation in HIV-infected patients.


