PNU 142633Highly selective 5-HT1D agonist CAS# 187665-65-2 |
- PF-562271
Catalog No.:BCC3674
CAS No.:717907-75-0
- TAE226 (NVP-TAE226)
Catalog No.:BCC3885
CAS No.:761437-28-9
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
- PF-00562271
Catalog No.:BCC3684
CAS No.:939791-38-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 187665-65-2 | SDF | Download SDF |
| PubChem ID | 9845148 | Appearance | Powder |
| Formula | C24H30N4O3 | M.Wt | 422.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
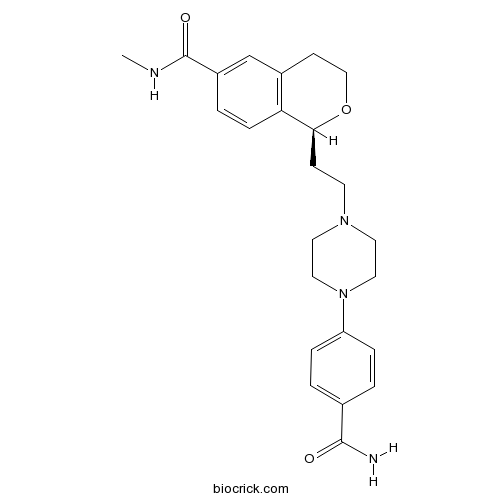
| Chemical Name | (1S)-1-[2-[4-(4-carbamoylphenyl)piperazin-1-yl]ethyl]-N-methyl-3,4-dihydro-1H-isochromene-6-carboxamide | ||
| SMILES | CNC(=O)C1=CC2=C(C=C1)C(OCC2)CCN3CCN(CC3)C4=CC=C(C=C4)C(=O)N | ||
| Standard InChIKey | PNTVCCRNJOGKGA-QFIPXVFZSA-N | ||
| Standard InChI | InChI=1S/C24H30N4O3/c1-26-24(30)19-4-7-21-18(16-19)9-15-31-22(21)8-10-27-11-13-28(14-12-27)20-5-2-17(3-6-20)23(25)29/h2-7,16,22H,8-15H2,1H3,(H2,25,29)(H,26,30)/t22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Very selective, high affinity 5-HT1D receptor agonist (Ki values are 6 and > 18000 nM at human 5-HT1D and 5-HT1B receptors respectively). Inhibits sympathetically-induced tachycardic responses and blocks neurogenic plasma protein extravasation in vivo. |

PNU 142633 Dilution Calculator

PNU 142633 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3667 mL | 11.8335 mL | 23.667 mL | 47.3339 mL | 59.1674 mL |
| 5 mM | 0.4733 mL | 2.3667 mL | 4.7334 mL | 9.4668 mL | 11.8335 mL |
| 10 mM | 0.2367 mL | 1.1833 mL | 2.3667 mL | 4.7334 mL | 5.9167 mL |
| 50 mM | 0.0473 mL | 0.2367 mL | 0.4733 mL | 0.9467 mL | 1.1833 mL |
| 100 mM | 0.0237 mL | 0.1183 mL | 0.2367 mL | 0.4733 mL | 0.5917 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PNU 109291
Catalog No.:BCC7408
CAS No.:187665-60-7
- Tataramide B
Catalog No.:BCN3897
CAS No.:187655-56-7
- Fmoc-D-Arg(Pbf)-OH
Catalog No.:BCC3077
CAS No.:187618-60-6
- Ethyl rutinoside
Catalog No.:BCC8976
CAS No.:187539-57-7
- MaxiPost
Catalog No.:BCC7984
CAS No.:187523-35-9
- N-Aminophthalimide
Catalog No.:BCC9085
CAS No.:1875-48-5
- Sitoindoside I
Catalog No.:BCN1161
CAS No.:18749-71-8
- Methylisopelletierine
Catalog No.:BCN1160
CAS No.:18747-42-7
- ER 50891
Catalog No.:BCC7783
CAS No.:187400-85-7
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Kimcuongin
Catalog No.:BCN7472
CAS No.:1872403-23-0
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Serpentine
Catalog No.:BCN1162
CAS No.:18786-24-8
- Arachidonyl serotonin
Catalog No.:BCC7500
CAS No.:187947-37-1
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
- Acetylcorynoline
Catalog No.:BCN1239
CAS No.:18797-80-3
- WKYMVM trifluoroacetate salt
Catalog No.:BCC5815
CAS No.:187986-11-4
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- Bikinin
Catalog No.:BCC5582
CAS No.:188011-69-0
- Abacavir sulfate
Catalog No.:BCC5023
CAS No.:188062-50-2
Safety and efficacy of PNU-142633, a selective 5-HT1D agonist, in patients with acute migraine.[Pubmed:11595000]
Cephalalgia. 2001 Sep;21(7):727-32.
In this randomized, double-blind, placebo-controlled, parallel-group study, patients received a single 50-mg oral dose of a 5-HT(1D) agonist, PNU-142633 (n = 34), or matching placebo (n = 35) during an acute migraine attack. No statistically significant treatment effects were observed at 1 and 2 h after dosing, even after stratifying by baseline headache intensity. At 1 and 2 h post-dose, 8.8% and 29.4% of the PNU-142633 group, respectively, and 8.6% and 40.0% of the placebo group, respectively, experienced headache relief; 2.9% and 8.8% of the PNU-142633 group and 0% and 5.7% of the placebo group were free of headache pain. Adverse events associated with PNU-142633 treatment included chest pain (two patients) and QTc prolongation (three patients). Results from this study suggest that anti-migraine efficacy is not mediated solely through the 5-HT(1D) receptor subtype, although this receptor may contribute, at least in part, to the adverse cardiovascular effects observed with 5-HT agonist medications.
Preclinical studies characterizing the anti-migraine and cardiovascular effects of the selective 5-HT1D receptor agonist PNU-142633.[Pubmed:12485205]
Cephalalgia. 2002 Dec;22(10):799-806.
The present study describes the preclinical pharmacology of a highly selective 5-HT1D receptor agonist PNU-142633. PNU-142633 binds with a Ki of 6 nm at the human 5-HT1D receptor and a Ki of> 18 000 nm at the human 5-HT1B receptor. The intrinsic activity of PNU-142633 at the human 5-HT1D receptor was determined to be 70% that of 5-HT in a cytosensor cell-based assay compared with 84% for that of sumatriptan. PNU-142633 was equally effective as sumatriptan and a half-log more potent than sumatriptan in preventing plasma protein extravasation induced by electrical stimulation of the trigeminal ganglion. Like sumatriptan, PNU-142633 reduced the increase in cat nucleus trigeminal caudalis blood flow elicited by electrical stimulation of the trigeminal ganglion compared with the vehicle control. The direct vasoconstrictor potential of PNU-142633 was evaluated in vascular beds. Sumatriptan increased vascular resistance in carotid, meningeal and coronary arteries while PNU-142633 failed to alter resistance in these vascular beds. These data are discussed in relation to the clinical findings of PNU-142633 in a phase II acute migraine study.
Pharmacological profile of the 5-HT-induced inhibition of cardioaccelerator sympathetic outflow in pithed rats: correlation with 5-HT1 and putative 5-ht5A/5B receptors.[Pubmed:14504136]
Br J Pharmacol. 2003 Oct;140(4):725-35.
Continuous infusions of 5-hydroxytryptamine (5-HT) inhibit the tachycardiac responses to preganglionic (C7-T1) sympathetic stimulation in pithed rats pretreated with desipramine. The present study identified the pharmacological profile of this inhibitory action of 5-HT. The inhibition induced by intravenous (i.v.) continuous infusions of 5-HT (5.6 microg x kg-1x min-1) on sympathetically induced tachycardiac responses remained unaltered after i.v. treatment with saline or the antagonists GR 127935 (5-HT1B/1D), the combination of WAY 100635 (5-HT1A) plus GR 127935, ritanserin (5-HT2), tropisetron (5-HT3/4), LY215840 (5-HT7) or a cocktail of antagonists/inhibitors consisting of yohimbine (alpha2), prazosin (alpha1), ritanserin, GR 127935, WAY 100635 and indomethacin (cyclooxygenase), but was abolished by methiothepin (5-HT1/2/6/7 and recombinant 5-ht5A/5B). These drugs, used in doses high enough to block their respective receptors/mechanisms, did not modify the sympathetically induced tachycardiac responses per se. I.v. continuous infusions of the agonists 5-carboxamidotryptamine (5-CT; 5-HT1/7 and recombinant 5-ht5A/5B), CP 93129 (r5-HT1B), sumatriptan (5-HT1B/1D), PNU-142633 (5-HT1D) and ergotamine (5-HT1B/1D and recombinant 5-ht5A/5B) mimicked the above sympatho-inhibition to 5-HT. In contrast, the agonists indorenate (5-HT1A) and LY344864 (5-ht1F) were inactive. Interestingly, 5-CT-induced cardiac sympatho-inhibition was abolished by methiothepin, the cocktail of antagonists/inhibitors, GR 127935 or the combination of SB224289 (5-HT1B) plus BRL15572 (5-HT1D), but remained unchanged when SB224289 or BRL15572 were given separately. Therefore, 5-HT-induced cardiac sympatho-inhibition, being unrelated to 5-HT2, 5-HT3, 5-HT4, 5-ht6, 5-HT7 receptors, alpha1/2-adrenoceptor or prostaglandin synthesis, seems to be primarily mediated by (i). 5-HT1 (probably 5-HT1B/1D) receptors and (ii). a novel mechanism antagonized by methiothepin that, most likely, involves putative 5-ht5A/5B receptors.


