BIO 1211α4β1 inhibitor,selective and high affinity CAS# 187735-94-0 |
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Cilengitide
Catalog No.:BCC3942
CAS No.:188968-51-6
- TR-14035
Catalog No.:BCC4266
CAS No.:232271-19-1
- BIO 5192
Catalog No.:BCC8002
CAS No.:327613-57-0
- Firategrast
Catalog No.:BCC1575
CAS No.:402567-16-2
- Zaurategrast
Catalog No.:BCC2070
CAS No.:455264-31-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 187735-94-0 | SDF | Download SDF |
| PubChem ID | 9961766 | Appearance | Powder |
| Formula | C36H48N6O9 | M.Wt | 708.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in 0.2M PBS | ||
| Sequence | MPUPA-LDVP | ||
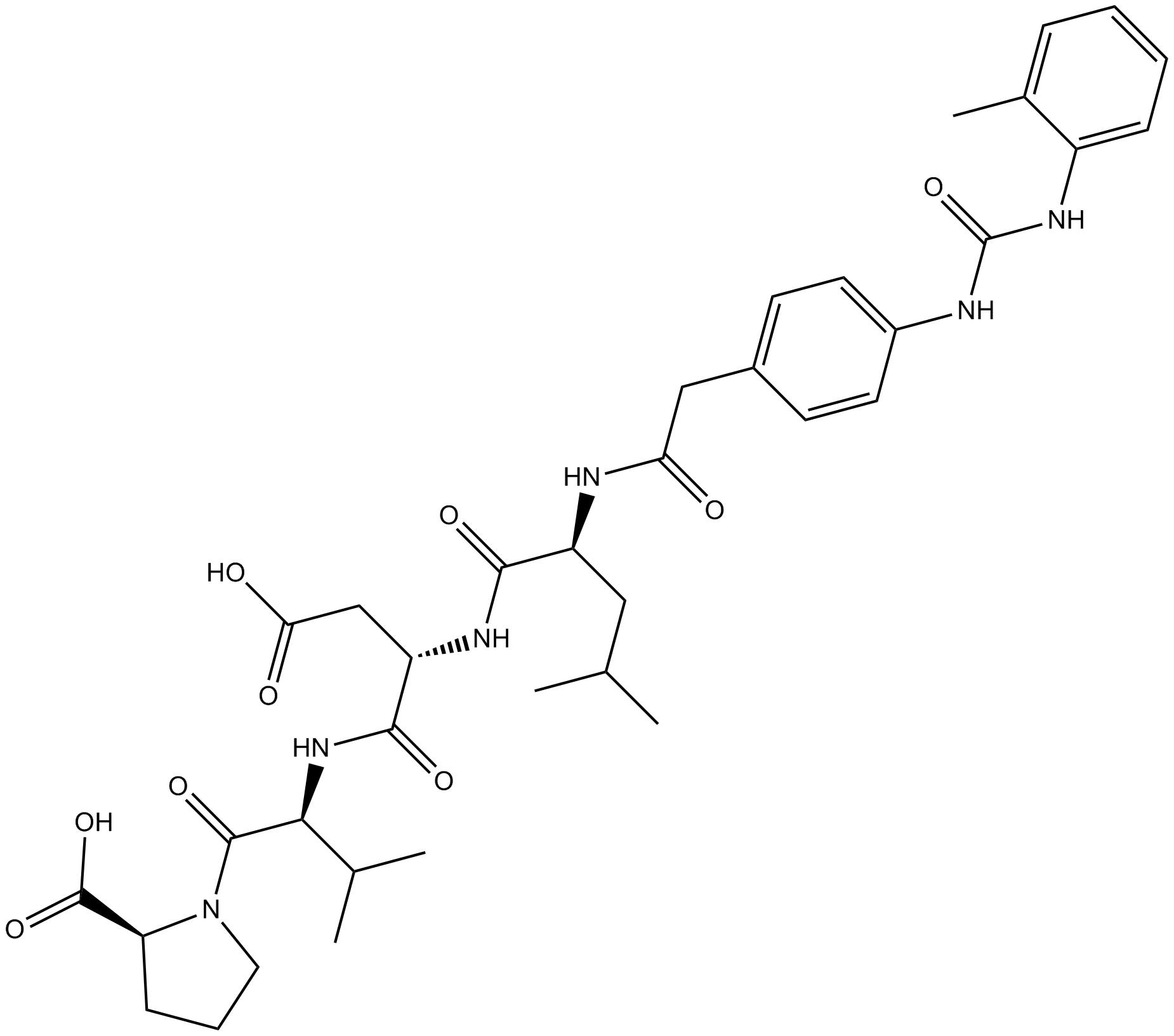
| Chemical Name | (2S)-1-[(2S)-2-[[(2S)-3-carboxy-2-[[(2S)-4-methyl-2-[[2-[4-[(2-methylphenyl)carbamoylamino]phenyl]acetyl]amino]pentanoyl]amino]propanoyl]amino]-3-methylbutanoyl]pyrrolidine-2-carboxylic acid | ||
| SMILES | CC1=CC=CC=C1NC(=O)NC2=CC=C(C=C2)CC(=O)NC(CC(C)C)C(=O)NC(CC(=O)O)C(=O)NC(C(C)C)C(=O)N3CCCC3C(=O)O | ||
| Standard InChIKey | NVVGCQABIHSJSQ-KFZSMJGVSA-N | ||
| Standard InChI | InChI=1S/C36H48N6O9/c1-20(2)17-26(38-29(43)18-23-12-14-24(15-13-23)37-36(51)40-25-10-7-6-9-22(25)5)32(46)39-27(19-30(44)45)33(47)41-31(21(3)4)34(48)42-16-8-11-28(42)35(49)50/h6-7,9-10,12-15,20-21,26-28,31H,8,11,16-19H2,1-5H3,(H,38,43)(H,39,46)(H,41,47)(H,44,45)(H,49,50)(H2,37,40,51)/t26-,27-,28-,31-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, high affinity α4β1 (Very Late Antigen 4; VLA-4) inhibitor; displays 200-fold selectivity for the activated form of α4β1 (KD = 70 pM; IC50 = 0.004 μM). Selective for α4β1 over a range of other integrins (IC50 >100 μM for α1β1, α5β1 and α6β1). |

BIO 1211 Dilution Calculator

BIO 1211 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BIO 1211 is a selective and high affinity inhibitor of α4β1 with IC50 value of 0.004uM [1].
The leukocyte integrin α4β1 mediates leukocyte recruitment, activation, apoptosis inhibition and mediator release, it plays an important role in inflammatory responses [1].
BIO-1211 was about 200-fold selective for the activated form of α4β1 and it stimulated expression of ligand-induced epitopes on the integrin β1 subunit, consistent with occupancy of the receptor’s ligand-binding site [1]. In Jurkat cells, KD values for BIO1211 binding ranged from 20–40 nM in the nonactivated state of the integrin to 100 pM in the activated state. Different KD values were controlled exclusively by differences in the dissociation rates of the integrin-BIO1211 complex [2].
Treatment allergic sheep with a 3-mg nebulized dose BIO-1211, BIO-1211 prevented nonspecific airway hyperresponsiveness to carbachol and inhibited early and late airway responses following antigen challenge [1].
References:
[1]. Lin Kc, Ateeq HS, Hsiung SH, et al. Selective, tight-binding inhibitors of integrin α4β1 that inhibit allergic airway responses. J Med Chem, 1999, 42(5): 920-934.
[2]. Chen LL, Whitty A, Lobb RR, et al. Multiple activation sites of integrin α4β1 detected through their different affinities for a small molecule ligand. J Biol Chem, 1999, 274(19): 13167-13175.
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
- PNU 142633
Catalog No.:BCC7222
CAS No.:187665-65-2
- PNU 109291
Catalog No.:BCC7408
CAS No.:187665-60-7
- Tataramide B
Catalog No.:BCN3897
CAS No.:187655-56-7
- Fmoc-D-Arg(Pbf)-OH
Catalog No.:BCC3077
CAS No.:187618-60-6
- Ethyl rutinoside
Catalog No.:BCC8976
CAS No.:187539-57-7
- MaxiPost
Catalog No.:BCC7984
CAS No.:187523-35-9
- N-Aminophthalimide
Catalog No.:BCC9085
CAS No.:1875-48-5
- Sitoindoside I
Catalog No.:BCN1161
CAS No.:18749-71-8
- Methylisopelletierine
Catalog No.:BCN1160
CAS No.:18747-42-7
- ER 50891
Catalog No.:BCC7783
CAS No.:187400-85-7
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- Serpentine
Catalog No.:BCN1162
CAS No.:18786-24-8
- Arachidonyl serotonin
Catalog No.:BCC7500
CAS No.:187947-37-1
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
- Acetylcorynoline
Catalog No.:BCN1239
CAS No.:18797-80-3
- WKYMVM trifluoroacetate salt
Catalog No.:BCC5815
CAS No.:187986-11-4
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- Bikinin
Catalog No.:BCC5582
CAS No.:188011-69-0
- Abacavir sulfate
Catalog No.:BCC5023
CAS No.:188062-50-2
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
[Inhibitory effects of BIO-1211 on bronchoconstriction and neutrophil adhesion in rats].[Pubmed:18705005]
Zhejiang Da Xue Xue Bao Yi Xue Ban. 2008 Jul;37(4):340-4.
OBJECTIVE: To determine the inhibitory effects of BIO-1211, a very late antigen-4 (vla-4) antagonist, on bronchoconstriction and neutrophil adhesion in rats. METHODS: For evaluating ovalbumin-induced bronchoconstriction in the sensitized rats, the changes in lung resistance (RL) and lung dynamic compliance (C(dyn)) were determined after antigen challenge. Neutrophils from the rats were used to determine fibronectin and serum-induced cell adhesion. The effect of BIO-1211 on wheezing was determined after inhalation of histamine and acetylcholine in guinea pigs. RESULT: BIO-1211 aerosol at 1, 3 and 10 mg/ml significantly inhibited the changes in lung resistance and lung dynamic compliance after antigen challenge in the sensitized rats in a dose-dependent manner. BIO-1211 at 25, 50, 100 and 200 microgram/ml inhibited the fibronectin-induced neutrophil adhesion by 23.5%, 24.6%, 61.4% and 58.1%, respectively, and serum-induced adhesion by 29.9%, 35.9%, 35.3% and 15.4%, respectively. Inhalation of 10 mg/ml BIO-1211 did not show any protection against histamine and acetylcholine-induced bronchoconstriction. CONCLUSION: BIO-1211 inhibits bronchoconstriction and neutrophil adhesion, which may be associated with its effect against bronchoconstriction in rats.
BIO-1211 (Biogen).[Pubmed:16100687]
IDrugs. 2000 May;3(5):536-40.
Biogen, in collaboration with Merck & Co, is developing late activator VLA-4 (alpha4beta1) integrin antagonists for the potential treatment of inflammatory conditions [271194]. Merck has begun phase II trials with the lead compound, BIO-1211, for asthma, Biogen is still conducting preclinical research for its designated indications [317648,319225]. Under the collaborative agreement, each company has worldwide rights to certain indications; Merck has rights for asthma and Biogen retains the rights to a number of smaller indications, including multiple sclerosis, inflammatory bowel disease, renal indications and most diseases in which the US patient population is less than 200,000 [271194]. VLA-4 inhibitors show anti-inflammatory action by inhibition of binding between adhesion factors and leukocytes, but with no loss of basophil function, and they have the advantage of specificity not seen with existing drugs [273417]. In February 1999, Lehman Brothers predicted 40% probabilities that the compound would reach the US and ex-US markets for the asthma indication (Merck), and launch onto these markets by 2003. Peak annual sales of US dollar 500 million (US) and US dollar 500 million (outside US) are predicted, both in 2010 [319225].
Prophylactic Effect of BIO-1211 Small-Molecule Antagonist of VLA-4 in the EAE Mouse Model of Multiple Sclerosis.[Pubmed:26436854]
Immunol Invest. 2015;44(7):694-712.
BACKGROUND AND PURPOSE: Some functional limitations and economic burden of therapeutic antibodies indicated that introducing of alternative therapeutic compounds with same or different mechanism of action could be worthwhile. In this regard small-molecule antagonists can have a wide range of impacts, so in this research, we examine the prophylactic effects of BIO-1211 [Very Late Antigen-4 (VLA4) blocker], in experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis in comparison with commercial available medicine, Natalizumab (NTZ)]. METHODS: EAE was induced by subcutaneous immunization of myelin oligodendrocyte glycoprotein (MOG35-55) in 8-week-old C57BL/6 mice. During EAE induction, mice were separated to distinct groups and provided either BIO-1211 (5 and 10 mg/kg) or NTZ (5 mg/kg) and co-administration of these two compounds. After 21 days, neuro-inflammatory responses were analyzed using qRT-PCR, western blot, and ELISA methods. Pervade of immune cells to brain was examined by Evans blue staining and immunohistochemistry (IHC) analysis of specific markers of microglia/monocytes (CD11b) and leukocytes (CD45). RESULTS: Targeted disruption of VLA4/VCAM1 interactions, by BIO-1211 agonist in mice, results in reduced cytokines expression, leukocyte trafficking, and inhibition of inflammatory responses in EAE (p < 0.01) in a dose-independent manner (data not shown). Mice treated with both BIO-1211 and NTZ exhibited a considerable depletion in the EAE clinical score, which correlated with decreased expression of TNF-alpha, IL-17, IFN-gamma and pervade of CD11b(+) and CD45(+) cells into the cerebral cortex. CONCLUSION: Our results indicated that BIO12-11 compound would be an useful tool to further understand the biological roles of VLA4/VCAM1 interactions, and could also be considered as EAE-suppressing agent.
[Effects of BIO-1211 on eosinophil chemotaxis, recruitment and mediator release].[Pubmed:12970925]
Zhejiang Da Xue Xue Bao Yi Xue Ban. 2003 Aug;32(4):279-82, 291.
OBJECTIVE: To study the effects of very late antigen(VLA) antagonist BIO-1211 on eosinophil chemotaxis, recruitment and mediator release. METHODS: Eosinophil chemotaxis was induced by platelet activating factor(PAF) in vitro and eosinophil recruitment and release were determined in vivo. RESULT: VLA antagonist BIO-1211 inhibited eosinophil chemotaxis induced by PAF. The inhibitory rates at 4x10(-11), 4x10(-10), 4x10(-9) mol x L(-1) were 24.9%, 29.9%, and 31.3%, respectively. Pretreatment by BIO-1211 1, 3 and 10 mg x kg(-1) intraperitoneally inhibited the recruitment of eosinophils in PAF in the rat induced by Sephadex in a dose dependent manner. Inhibitory rates were 60.3%, 68.9%, and 72.9%(P<0.05), respectively. BIO-1211 did not inhibit eosinophil peroxidase(EPO) release from eosinophils. CONCLUSION: BIO-1211 inhibits eosinophil chemotaxis and recruitment, alleviates local inflammation, and may represent a new type of drug for allergic diseases.
Discovery of trans-4-[1-[[2,5-Dichloro-4-(1-methyl-3-indolylcarboxamido)phenyl]acetyl]-(4S)-me thoxy-(2S)-pyrrolidinylmethoxy]cyclohexanecarboxylic acid: an orally active, selective very late antigen-4 antagonist.[Pubmed:19891440]
J Med Chem. 2009 Dec 24;52(24):7974-92.
We have focused on optimization of the inadequate pharmacokinetic profile of trans-4-substituted cyclohexanecarboxylic acid 5, which is commonly observed in many small molecule very late antigen-4 (VLA-4) antagonists. We modified the lipophilic moiety in 5 and found that reducing the polar surface area of this moiety results in improvement of the PK profile. Consequently, our efforts have led to the discovery of trans-4-[1-[[2,5-dichloro-4-(1-methyl-3-indolylcarboxamido)phenyl]acetyl]-(4S)-me thoxy-(2S)-pyrrolidinylmethoxy]cyclohexanecarboxylic acid (14e) with potent activity (IC(50) = 5.4 nM) and significantly improved bioavailability in rats, dogs, and monkeys (100%, 91%, 68%), which demonstrated excellent oral efficacy in murine and guinea pig models of asthma. Based on its overall profile, compound 14e was progressed into clinical trails. In a single ascending-dose phase I clinical study, compound 14e exhibited favorable oral exposure as expected and had no serious adverse events.
Multiple activation states of integrin alpha4beta1 detected through their different affinities for a small molecule ligand.[Pubmed:10224072]
J Biol Chem. 1999 May 7;274(19):13167-75.
We have used the highly specific alpha4beta1 inhibitor 4-((N'-2-methylphenyl)ureido)-phenylacetyl-leucine-aspartic acid-valine-proline (BIO1211) as a model LDV-containing ligand to study alpha4beta1 integrin-ligand interactions on Jurkat cells under diverse conditions that affect the activation state of alpha4beta1. Observed KD values for BIO1211 binding ranged from a value of 20-40 nM in the non-activated state of the integrin that exists in 1 mM Mg2+, 1 mM Ca2+ to 100 pM in the activated state seen in 2 mM Mn2+ to 18 pM when binding was measured after co-activation by 2 mM Mn2+ plus 10 microgram/ml of the integrin-activating monoclonal antibody TS2/16. The large range in KD values was governed almost exclusively by differences in the dissociation rates of the integrin-BIO1211 complex, which ranged from 0.17 x 10(-4) s-1 to >140 x 10(-4) s-1. Association rate constants varied only slightly under the same conditions, all falling in the narrow range from 0.9 to 2.7 x 10(6) M-1 s-1. The further increase in affinity observed upon co-activation by divalent cations and TS2/16 compared with that observed at saturating concentrations of metal ions or TS2/16 alone indicates that the mechanism by which these factors bring about activation are distinct and identified a previously unrecognized high affinity state on alpha4beta1 that had not been detected by conventional assay methods. Similar changes in affinity were observed when the binding properties of vascular cell adhesion molecule-1 and CS1 to alpha4beta1 were studied, indicating that the different affinity states detected with BIO1211 are an inherent property of the integrin.
Selective, tight-binding inhibitors of integrin alpha4beta1 that inhibit allergic airway responses.[Pubmed:10072689]
J Med Chem. 1999 Mar 11;42(5):920-34.
Integrin alpha4beta1 mediates leukocyte recruitment, activation, mediator release, and apoptosis inhibition, and it plays a central role in inflammatory pathophysiology. High-affinity, selective inhibitors of alpha4beta1, based on the Leu-Asp-Val (LDV) sequence from the alternatively spliced connecting segment-1 (CS-1) peptide of cellular fibronectin, are described that employ a novel N-terminal peptide "cap" strategy. One inhibitor, BIO-1211, was approximately 10(6)-fold more potent than the starting peptide and exhibited tight-binding properties (koff = 1.4 x 10(-4) s-1, KD = 70 pM), a remarkable finding for a noncovalent, small-molecule inhibitor of a protein receptor. BIO-1211 was also 200-fold selective for the activated form of alpha4beta1, and it stimulated expression of ligand-induced epitopes on the integrin beta1 subunit, a property consistent with occupancy of the receptor's ligand-binding site. Pretreatment of allergic sheep with a 3-mg nebulized dose of BIO-1211 inhibited early and late airway responses following antigen challenge and prevented development of nonspecific airway hyperresponsiveness to carbachol. These results show that highly selective and potent small-molecule antagonists can be identified to integrins with primary specificity for peptide domains other than Arg-Gly-Asp (RGD); they confirm the generality of integrins as small molecule targets; and they validate alpha4beta1 as a therapeutic target for asthma.


